Plasma brain-derived tau is an amyloid-associated neurodegeneration biomarker in Alzheimer's disease
- PMID: 38575616
- PMCID: PMC10995141
- DOI: 10.1038/s41467-024-47286-5
Plasma brain-derived tau is an amyloid-associated neurodegeneration biomarker in Alzheimer's disease
Abstract
Staging amyloid-beta (Aβ) pathophysiology according to the intensity of neurodegeneration could identify individuals at risk for cognitive decline in Alzheimer's disease (AD). In blood, phosphorylated tau (p-tau) associates with Aβ pathophysiology but an AD-type neurodegeneration biomarker has been lacking. In this multicenter study (n = 1076), we show that brain-derived tau (BD-tau) in blood increases according to concomitant Aβ ("A") and neurodegeneration ("N") abnormalities (determined using cerebrospinal fluid biomarkers); We used blood-based A/N biomarkers to profile the participants in this study; individuals with blood-based p-tau+/BD-tau+ profiles had the fastest cognitive decline and atrophy rates, irrespective of the baseline cognitive status. Furthermore, BD-tau showed no or much weaker correlations with age, renal function, other comorbidities/risk factors and self-identified race/ethnicity, compared with other blood biomarkers. Here we show that blood-based BD-tau is a biomarker for identifying Aβ-positive individuals at risk of short-term cognitive decline and atrophy, with implications for clinical trials and implementation of anti-Aβ therapies.
© 2024. The Author(s).
Conflict of interest statement
M.T. and P.H. are employees of Bioventix Plc. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, and has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche. K.B. has served as a consultant and at advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, BioArctic, Celdara Medical, Eisai and Roche Diagnostics. H.Z. and K.B. are co-founders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. M.S.C. has served as a consultant and at advisory boards for Roche Diagnostics International Ltd and Grifols S.L., has given lectures in symposia sponsored by Roche Diagnostics, S.L.U. and Roche Farma, S.A., and was granted with a project funded by Roche Diagnostics International Ltd; payments were made to the institution (BBRC). A.P.P. has served on advisory boards for Schwabe Farma Iberica. T.K.K. has received honoraria from the University of Wisconsin Madison and the University of Pennsylvania and has an awarded patent (#WO2020193500A1), all unrelated to this work. B.E.K. has served as a consultant for Biogen and advisory board for Eisai. T.F. has served as a consultant and at the advisory boards for Biogen, Novo Nordisk, Eli Lilly, and Roche. The other authors declare no competing interest.
Figures

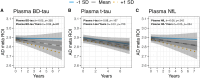
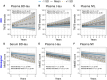

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG075336/AG/NIA NIH HHS/United States
- R01 AG083874/AG/NIA NIH HHS/United States
- R01 AG072641/AG/NIA NIH HHS/United States
- R01 AG058969/AG/NIA NIH HHS/United States
- RF1 AG025516/AG/NIA NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
- R37 AG023651/AG/NIA NIH HHS/United States
- U24 AG082930/AG/NIA NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- R01 AG073267/AG/NIA NIH HHS/United States
- R01 AG025516/AG/NIA NIH HHS/United States
- RF1 AG052525/AG/NIA NIH HHS/United States
- R01 AG053952/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

