Crosstalk between metabolism and cell death in tumorigenesis
- PMID: 38575922
- PMCID: PMC10993426
- DOI: 10.1186/s12943-024-01977-1
Crosstalk between metabolism and cell death in tumorigenesis
Abstract
It is generally recognized that tumor cells proliferate more rapidly than normal cells. Due to such an abnormally rapid proliferation rate, cancer cells constantly encounter the limits of insufficient oxygen and nutrient supplies. To satisfy their growth needs and resist adverse environmental events, tumor cells modify the metabolic pathways to produce both extra energies and substances required for rapid growth. Realizing the metabolic characters special for tumor cells will be helpful for eliminating them during therapy. Cell death is a hot topic of long-term study and targeting cell death is one of the most effective ways to repress tumor growth. Many studies have successfully demonstrated that metabolism is inextricably linked to cell death of cancer cells. Here we summarize the recently identified metabolic characters that specifically impact on different types of cell deaths and discuss their roles in tumorigenesis.
Keywords: Apoptosis; Autophage; Cell death; Cuproptosis; Ferroptosis; Pyroptosis; Tumor metabolism; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
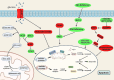
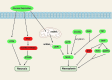

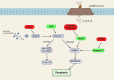


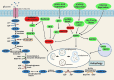
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

