Alternative consent methods used in the multinational, pragmatic, randomised clinical trial SafeBoosC-III
- PMID: 38575977
- PMCID: PMC10996265
- DOI: 10.1186/s13063-024-08074-0
Alternative consent methods used in the multinational, pragmatic, randomised clinical trial SafeBoosC-III
Abstract
Background: The process of obtaining prior informed consent for experimental treatment does not fit well into the clinical reality of acute and intensive care. The therapeutic window of interventions is often short, which may reduce the validity of the consent and the rate of enrolled participants, to delay trial completion and reduce the external validity of the results. Deferred consent and 'opt-out' are alternative consent methods. The SafeBoosC-III trial was a randomised clinical trial investigating the benefits and harms of cerebral oximetry monitoring in extremely preterm infants during the first 3 days after birth, starting within the first 6 h after birth. Prior, deferred and opt-out consent were all allowed by protocol. This study aimed to evaluate the use of different consent methods in the SafeBoosC-III trial, Furthermore, we aimed to describe and analyse concerns or complaints that arose during the first 6 months of trial conduct.
Methods: All 70 principal investigators were invited to join this descriptive ancillary study. Each principal investigator received a questionnaire on the use of consent methods in their centre during the SafeBoosC-III trial, including the possibility to describe any concerns related to the consent methods used during the first 6 months of the trial, as raised by the parents or the clinical staff.
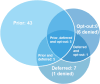
Results: Data from 61 centres were available. In 43 centres, only prior informed consent was used: in seven, only deferred consent. No centres used the opt-out method only, but five centres used prior and deferred, five used prior, deferred and opt-out (all possibilities) and one used both deferred and opt-out. Six centres applied to use the opt-out method by their local research ethics committee but were denied using it. One centre applied to use deferred consent but was denied. There were only 23 registered concerns during the execution of the trial.
Conclusions: Consent by opt-out was allowed by the protocol in this multinational trial but only a few investigators opted for it and some research ethics boards did not accept its use. It is likely to need promotion by the clinical research community to unfold its potential.
Keywords: Consent; Deferred consent; Ethic; Neonatal; Opt-out consent; Prior consent; Trial.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interest
Figures
References
-
- European Medicines Agency C for H, Products. M. Guideline on Good Clinical Practice E6(R2). 2017
-
- McIntosh N, Bates P, Brykczynska G, Dunstan G, Goldman A, Harvey D, et al. Guidelines for the ethical conduct of medical research involving children. Royal College of Paediatrics, Child Health: Ethics Advisory Committee. Arch Dis Child [Internet]. 2000 [cited 2024 Mar 19];82(2):177–82. Available from: https://pubmed.ncbi.nlm.nih.gov/10648379/ - PMC - PubMed
-
- Gale C, Hyde MJ, Modi N. Research ethics committee decision-making in relation to an efficient neonatal trial. Arch Dis Child Fetal Neonatal Ed [Internet]. 2017 Jul 1 [cited 2024 Mar 19];102(4):F291–8. Available from: https://pubmed.ncbi.nlm.nih.gov/27630188/ - PMC - PubMed