Therapy-induced senescent tumor cell-derived extracellular vesicles promote colorectal cancer progression through SERPINE1-mediated NF-κB p65 nuclear translocation
- PMID: 38576002
- PMCID: PMC10993572
- DOI: 10.1186/s12943-024-01985-1
Therapy-induced senescent tumor cell-derived extracellular vesicles promote colorectal cancer progression through SERPINE1-mediated NF-κB p65 nuclear translocation
Abstract
Background: Cellular senescence frequently occurs during anti-cancer treatment, and persistent senescent tumor cells (STCs) unfavorably promote tumor progression through paracrine secretion of the senescence-associated secretory phenotype (SASP). Extracellular vesicles (EVs) have recently emerged as a novel component of the SASP and primarily mediate the tumor-promoting effect of the SASP. Of note, the potential effect of EVs released from STCs on tumor progression remains largely unknown.
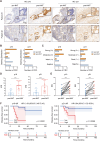
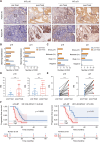
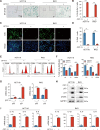
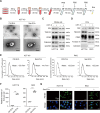
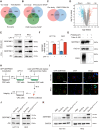
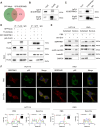
Methods: We collected tumor tissues from two cohorts of colorectal cancer (CRC) patients to examine the expression of p16, p21, and SERPINE1 before and after anti-cancer treatment. Cohort 1 included 22 patients with locally advanced rectal cancer (LARC) who received neoadjuvant therapy before surgical resection. Cohort 2 included 30 patients with metastatic CRC (mCRC) who received first-line irinotecan-contained treatment. CCK-8, transwell, wound-healing assay, and tumor xenograft experiments were carried out to determine the impacts of EVs released from STCs on CRC progression in vitro and in vivo. Quantitative proteomic analysis was applied to identify protein cargo inside EVs secreted from STCs. Immunoprecipitation and mass spectrometer identification were utilized to explore the binding partners of SERPINE1. The interaction of SERPINE1 with p65 was verified by co-immunoprecipitation, and their co-localization was confirmed by immunofluorescence.
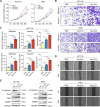
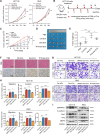
Results: Chemotherapeutic agents and irradiation could potently induce senescence in CRC cells in vitro and in human CRC tissues. The more significant elevation of p16 and p21 expression in patients after anti-cancer treatment displayed shorter disease-free survival (DFS) for LARC or progression-free survival (PFS) for mCRC. We observed that compared to non-STCs, STCs released an increased number of EVs enriched in SERPINE1, which further promoted the progression of recipient cancer cells. Targeting SERPINE1 with a specific inhibitor, tiplaxtinin, markedly attenuated the tumor-promoting effect of STCs-derived EVs. Additionally, the patients with greater increment of SERPINE1 expression after anti-cancer treatment had shorter DFS for LARC or PFS for mCRC. Mechanistically, SERPINE1 bound to p65, promoting its nuclear translocation and subsequently activating the NF-κB signaling pathway.
Conclusions: We provide the in vivo evidence of the clinical prognostic implications of therapy-induced senescence. Our results revealed that STCs were responsible for CRC progression by producing large amounts of EVs enriched in SERPINE1. These findings further confirm the crucial role of therapy-induced senescence in tumor progression and offer a potential therapeutic strategy for CRC treatment.
Keywords: Cellular senescence; Colorectal cancer; Extracellular vesicles; SERPINE1; p65.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
- cstc2019jcyj-msxmX0617/Natural Science Foundation Project of Chongqing, Chongqing Science and Technology Commission
- ZNJC202006/Translational Medicine and Interdisciplinary Research Joint Fund of Zhongnan Hospital of Wuhan University
- KYXM2023004/2022 Annual Funding for Discipline Construction, Zhongnan Hospital of Wuhan University
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

