Efficacy and safety of immunosuppressive therapy combined with eltrombopag for severe aplastic anemia: a systematic review and meta-analysis
- PMID: 38576005
- PMCID: PMC10993616
- DOI: 10.1186/s13643-024-02515-2
Efficacy and safety of immunosuppressive therapy combined with eltrombopag for severe aplastic anemia: a systematic review and meta-analysis
Abstract
Background and objective: Immunosuppressive therapy (IST) is the first choice for severe aplastic anemia (SAA) patients with hematopoietic stem cell transplantation (HSCT) limitation, and the main factor limiting its efficacy is too few residual hematopoietic stem/progenitor cells (HSPC). Eltrombopag (EPAG), as a small molecule thrombopoietin receptor agonist, can stimulate the proliferation of residual HSPC and restore the bone marrow hematopoietic function of patients. In recent years, many studies have observed the efficacy and safety of IST combined with EPAG in the treatment of SAA, but the results are still controversial. The aim of this study is to systematically evaluate the efficacy and safety of IST combined with or without EPGA in the treatment of SAA.
Methods: We conducted a systematic review of all relevant literature published up to January 19, 2024. Pooled odds ratio (OR) was calculated to compare the rates, along with 95% confidence intervals (CI) and p value to assess whether the results were statistically significant by Review Manager 5.4.1. The p values for the interactions between each subgroup were calculated by Stata 15.1. The Newcastle-Ottawa Scale and the Cochrane bias risk assessment tools were respectively used to evaluate the quality of the literature with cohort studies and randomized controlled trials. The Review Manager 5.4.1 and Stata 15.1 were used to assess bias risk and perform the meta-analysis.
Results: A total of 16 studies involving 2148 patients were included. The IST combined with the EPAG group had higher overall response rate (ORR) than the IST group at 3 months (pooled OR = 2.10, 95% CI 1.58-2.79, p < 0.00001) and 6 months (pooled OR = 2.13, 95% CI 1.60-2.83, p < 0.00001), but the difference between the two groups became statistically insignificant at 12 months (pooled OR = 1.13, 95% CI 0.75-1.72, p = 0.55). The results of complete response rate (CRR) (pooled OR at 3 months = 2.73, 95% CI 1.83-4.09, p < 0.00001, 6 months = 2.76, 95% CI 2.08-3.67, p < 0.00001 and 12 months = 1.38, 95% CI 0.85-2.23, p = 0.19) were similar to ORR. Compared with the IST group, the IST combined with the EPAG group had better overall survival rate (OSR) (pooled OR = 1.70, 95% CI 1.15-2.51, p = 0.008), but there were no statistically significant differences in event-free survival rate (EFSR) (pooled OR = 1.40, 95% CI 0.93-2.13, p = 0.11), clonal evolution rate (pooled OR = 0.68, 95% CI 0.46-1.00, p = 0.05) and other adverse events between the two groups. The results of subgroup analysis showed that different ages were a source of heterogeneity, but different study types and different follow-up times were not. Moreover, all p-values for the interactions were greater than 0.05, suggesting that the treatment effect was not influenced by subgroup characteristics.
Conclusion: EPAG added to IST enables patients to achieve earlier and faster hematologic responses with a higher rate of complete response. Although it had no effect on overall EFSR, it improved OSR and did not increase the incidence of clonal evolution and other adverse events.
© 2024. The Author(s).
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures
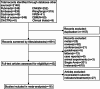









Similar articles
-
Thrombopoietin mimetics for patients with myelodysplastic syndromes.Cochrane Database Syst Rev. 2017 Sep 30;9(9):CD009883. doi: 10.1002/14651858.CD009883.pub2. Cochrane Database Syst Rev. 2017. PMID: 28962071 Free PMC article.
-
First-line allogeneic hematopoietic stem cell transplantation of HLA-matched sibling donors compared with first-line ciclosporin and/or antithymocyte or antilymphocyte globulin for acquired severe aplastic anemia.Cochrane Database Syst Rev. 2013 Jul 23;2013(7):CD006407. doi: 10.1002/14651858.CD006407.pub2. Cochrane Database Syst Rev. 2013. PMID: 23881658 Free PMC article.
-
Comparison of haploidentical-hematopoietic stem cell transplantation using modified post-transplantation cyclophosphamide and eltrombopag combined with immunosuppression for the treatment of severe aplastic anemia.Transplant Cell Ther. 2025 Aug 9:S2666-6367(25)01331-4. doi: 10.1016/j.jtct.2025.07.019. Online ahead of print. Transplant Cell Ther. 2025. PMID: 40789513
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Rhino-orbito-cerebral mucormycosis after allogeneic hematopoietic stem cell transplantation for very severe aplastic anemia in a child: a case report.Front Pediatr. 2025 Apr 14;13:1529656. doi: 10.3389/fped.2025.1529656. eCollection 2025. Front Pediatr. 2025. PMID: 40297559 Free PMC article.
-
Whole Exome Sequencing of Adult Indians with Apparently Acquired Aplastic Anaemia: Initial Experience at Tertiary Care Hospital.Diseases. 2024 Sep 23;12(9):225. doi: 10.3390/diseases12090225. Diseases. 2024. PMID: 39329894 Free PMC article.
References
-
- Liu L, Lei M, Fu R, Han B, Zhao X, Liu R, et al. Matched related transplantation versus immunosuppressive therapy plus eltrombopag for first-line treatment of severe aplastic anemia: a multicenter, prospective study. J Hematol Oncol. 2022;15:105. doi: 10.1186/s13045-022-01324-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

