Application of deep learning on mammographies to discriminate between low and high-risk DCIS for patient participation in active surveillance trials
- PMID: 38576031
- PMCID: PMC10996224
- DOI: 10.1186/s40644-024-00691-x
Application of deep learning on mammographies to discriminate between low and high-risk DCIS for patient participation in active surveillance trials
Abstract
Background: Ductal Carcinoma In Situ (DCIS) can progress to invasive breast cancer, but most DCIS lesions never will. Therefore, four clinical trials (COMET, LORIS, LORETTA, AND LORD) test whether active surveillance for women with low-risk Ductal carcinoma In Situ is safe (E. S. Hwang et al., BMJ Open, 9: e026797, 2019, A. Francis et al., Eur J Cancer. 51: 2296-2303, 2015, Chizuko Kanbayashi et al. The international collaboration of active surveillance trials for low-risk DCIS (LORIS, LORD, COMET, LORETTA), L. E. Elshof et al., Eur J Cancer, 51, 1497-510, 2015). Low-risk is defined as grade I or II DCIS. Because DCIS grade is a major eligibility criteria in these trials, it would be very helpful to assess DCIS grade on mammography, informed by grade assessed on DCIS histopathology in pre-surgery biopsies, since surgery will not be performed on a significant number of patients participating in these trials.
Objective: To assess the performance and clinical utility of a convolutional neural network (CNN) in discriminating high-risk (grade III) DCIS and/or Invasive Breast Cancer (IBC) from low-risk (grade I/II) DCIS based on mammographic features. We explored whether the CNN could be used as a decision support tool, from excluding high-risk patients for active surveillance.
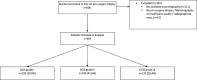
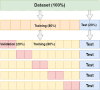
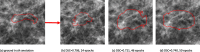
Methods: In this single centre retrospective study, 464 patients diagnosed with DCIS based on pre-surgery biopsy between 2000 and 2014 were included. The collection of mammography images was partitioned on a patient-level into two subsets, one for training containing 80% of cases (371 cases, 681 images) and 20% (93 cases, 173 images) for testing. A deep learning model based on the U-Net CNN was trained and validated on 681 two-dimensional mammograms. Classification performance was assessed with the Area Under the Curve (AUC) receiver operating characteristic and predictive values on the test set for predicting high risk DCIS-and high-risk DCIS and/ or IBC from low-risk DCIS.
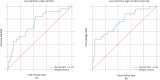
Results: When classifying DCIS as high-risk, the deep learning network achieved a Positive Predictive Value (PPV) of 0.40, Negative Predictive Value (NPV) of 0.91 and an AUC of 0.72 on the test dataset. For distinguishing high-risk and/or upstaged DCIS (occult invasive breast cancer) from low-risk DCIS a PPV of 0.80, a NPV of 0.84 and an AUC of 0.76 were achieved.
Conclusion: For both scenarios (DCIS grade I/II vs. III, DCIS grade I/II vs. III and/or IBC) AUCs were high, 0.72 and 0.76, respectively, concluding that our convolutional neural network can discriminate low-grade from high-grade DCIS.
Keywords: Active surveillance; Artificial intelligence; DCIS; DCIS grade; Deep learning; Invasive breast cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Cancer Research UK. (2017). [Online]. Available: http://www.cancerresearchuk.org/health-professional/cancer-statistics/st....
-
- Kankerbestrijding KWF. Incidentie- en overlevingscijfers: Nederlandse Kankerregistratie, februari 2016. [Online]. Available: https://www.kwf.nl/kanker/borstkanker.
-
- Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005. 10.1002/cncr.21069. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

