Vaginal Progesterone to Prevent Spontaneous Preterm Birth in Women With a Sonographic Short Cervix: The Story of the PREGNANT Trial
- PMID: 38576410
- PMCID: PMC11047312
- DOI: 10.1097/GRF.0000000000000867
Vaginal Progesterone to Prevent Spontaneous Preterm Birth in Women With a Sonographic Short Cervix: The Story of the PREGNANT Trial
Abstract
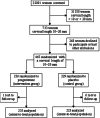
The PREGNANT trial was a randomized, placebo-controlled, multicenter trial designed to determine the efficacy and safety of vaginal progesterone (VP) to reduce the risk of birth < 33 weeks and of neonatal complications in women with a sonographic short cervix (10 to 20 mm) in the mid-trimester (19 to 23 6/7 wk). Patients allocated to receive VP had a 45% lower rate of preterm birth (8.9% vs 16.1%; relative risk = 0.55; 95% CI: 0.33-0.92). Neonates born to mothers allocated to VP had a 60% reduction in the rate of respiratory distress syndrome. This article reviews the background, design, execution, interpretation, and impact of the PREGNANT Trial.
Figures


Similar articles
-
Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial.Ultrasound Obstet Gynecol. 2011 Jul;38(1):18-31. doi: 10.1002/uog.9017. Epub 2011 Jun 15. Ultrasound Obstet Gynecol. 2011. PMID: 21472815 Free PMC article. Clinical Trial.
-
Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications.Am J Obstet Gynecol. 2016 Feb;214(2):235-242. doi: 10.1016/j.ajog.2015.09.102. Epub 2015 Oct 9. Am J Obstet Gynecol. 2016. PMID: 26450404 Free PMC article.
-
Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis.Am J Obstet Gynecol. 2018 Jul;219(1):10-25. doi: 10.1016/j.ajog.2018.03.028. Epub 2018 Apr 7. Am J Obstet Gynecol. 2018. PMID: 29630885 Free PMC article.
-
Re-analysis of the PREGNANT trial confirms that vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix.Ultrasound Obstet Gynecol. 2014 May;43(5):596-7. doi: 10.1002/uog.13331. Epub 2014 Apr 7. Ultrasound Obstet Gynecol. 2014. PMID: 24585456 No abstract available.
-
Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta-analysis.BJOG. 2015 Jan;122(1):27-37. doi: 10.1111/1471-0528.13032. Epub 2014 Aug 22. BJOG. 2015. PMID: 25145491 Free PMC article. Review.
Cited by
-
Neonatal Outcomes after Maternal Biomarker-Guided Preterm Birth Intervention: The AVERT PRETERM Trial.Diagnostics (Basel). 2024 Jul 9;14(14):1462. doi: 10.3390/diagnostics14141462. Diagnostics (Basel). 2024. PMID: 39061599 Free PMC article.
-
Treatment of cervical insufficiency and/or a short cervix with antimicrobial agents can restore cervical length and lead to pregnancy prolongation and term delivery.J Matern Fetal Neonatal Med. 2024 Dec;37(1):2349789. doi: 10.1080/14767058.2024.2349789. Epub 2024 May 15. J Matern Fetal Neonatal Med. 2024. PMID: 38749767 Free PMC article. No abstract available.
References
-
- Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–686. - PubMed
-
- Allen WM, Wintersteiner O. Crystalline progestin. Science. 1934;80:190–191. - PubMed
-
- Allen WM, Butenandt A, Corner GW, et al. . Nomenclature of corpus luteum hormone. Science. 1935;82:153. - PubMed
-
- Frobenius W. Ludwig Fraenkel, corpus luteum and discovery of progesterone. Zentralbl Gynakol. 1998;120:317–323. - PubMed

