SARS-CoV-2 infection of endothelial cells, dependent on flow-induced ACE2 expression, drives hypercytokinemia in a vascularized microphysiological system
- PMID: 38576426
- PMCID: PMC10991679
- DOI: 10.3389/fcvm.2024.1360364
SARS-CoV-2 infection of endothelial cells, dependent on flow-induced ACE2 expression, drives hypercytokinemia in a vascularized microphysiological system
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for COVID-19, has caused nearly 7 million deaths worldwide. Severe cases are marked by an aggressive inflammatory response known as hypercytokinemia, contributing to endothelial damage. Although vaccination has reduced hospitalizations, hypercytokinemia persists in breakthrough infections, emphasizing the need for disease models mimicking this response. Using a 3D microphysiological system (MPS), we explored the vascular role in SARS-CoV-2-induced hypercytokinemia.
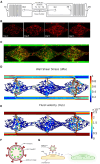
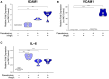
Methods: The vascularized micro-organ (VMO) MPS, consisting of human-derived primary endothelial cells (ECs) and stromal cells within an extracellular matrix, was used to model SARS-CoV-2 infection. A non-replicative pseudotyped virus fused to GFP was employed, allowing visualization of viral entry into human ECs under physiologic flow conditions. Expression of ACE2, TMPRSS2, and AGTR1 was analyzed, and the impact of viral infection on ACE2 expression, vascular inflammation, and vascular morphology was assessed.
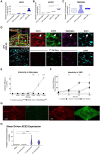
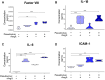
Results: The VMO platform facilitated the study of COVID-19 vasculature infection, revealing that ACE2 expression increased significantly in direct response to shear stress, thereby enhancing susceptibility to infection by pseudotyped SARS-CoV-2. Infected ECs secreted pro-inflammatory cytokines, including IL-6 along with coagulation factors. Cytokines released by infected cells were able to activate downstream, non-infected EC, providing an amplification mechanism for inflammation and coagulopathy.
Discussion: Our findings highlight the crucial role of vasculature in COVID-19 pathogenesis, emphasizing the significance of flow-induced ACE2 expression and subsequent inflammatory responses. The VMO provides a valuable tool for studying SARS-CoV-2 infection dynamics and evaluating potential therapeutics.
Keywords: COVID-19; endothelial dysfunction; hypercytokinemia; microphysiological systems; shear stress.
© 2024 Hatch, Piombo, Fang, Gach, Ewald, Van Trigt, Coon, Tong, Forthal and Hughes.
Conflict of interest statement
CCWH is a founder of, and has an equity interest in, Aracari Biosciences, Inc., which is commercializing the vascularized microtissue model. All work is with the full knowledge and approval of the UCI Conflict of Interest Oversight Committee. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

