Site-selective fatty acid chain conjugation of the N-terminus of the recombinant human granulocyte colony-stimulating factor
- PMID: 38576447
- PMCID: PMC10993259
- DOI: 10.3389/fbioe.2024.1360506
Site-selective fatty acid chain conjugation of the N-terminus of the recombinant human granulocyte colony-stimulating factor
Abstract
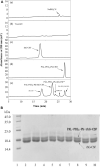
The clinical application of the recombinant human granulocyte colony-stimulating factor (rhG-CSF) is restricted by its short serum half-life. Herein, site-selective modification of the N-terminus of rhG-CSF with PAL-PEG3-Ph-CHO was used to develop a long-acting rhG-CSF. The optimized conditions for rhG-CSF modification with PAL-PEG3-Ph-CHO were: reaction solvent system of 3% (w/v) Tween 20 and 30 mM NaCNBH3 in acetate buffer (20 mmol/L, pH 5.0), molar ratio of PAL-PEG3-Ph-CHO to rhG-CSF of 6:1, temperature of 20°C, and reaction time of 12 h, consequently, achieving a PAL-PEG3-Ph-rhG-CSF product yield of 70.8%. The reaction mixture was purified via preparative liquid chromatography, yielding the single-modified product PAL-PEG3-Ph-rhG-CSF with a HPLC purity exceeding 95%. The molecular weight of PAL-PEG3-Ph-rhG-CSF was 19297 Da by MALDI-TOF-MS, which was consistent with the theoretical value. The circular dichroism analysis revealed no significant change in its secondary structure compared to unmodified rhG-CSF. The PAL-PEG3-Ph-rhG-CSF retained 82.0% of the in vitro biological activity of unmodified rhG-CSF. The pharmacokinetic analyses showed that the serum half-life of PAL-PEG3-Ph-rhG-CSF was 7.404 ± 0.777 h in mice, 4.08 times longer than unmodified rhG-CSF. Additionally, a single subcutaneous dose of PAL-PEG3-Ph-rhG-CSF presented comparable in vivo efficacy to multiple doses of rhG-CSF. This study demonstrated an efficacious strategy for developing long-acting rhG-CSF drug candidates.
Keywords: fatty acid chain conjugation; long-acting rhG-CSF; recombinant human granulocyte colony-stimulating factor; rhG-CSF; serum half-life; site-selective modification.
Copyright © 2024 Wang, Su, Du, Yu and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources

