Physiologically based modeling of LNP-mediated delivery of mRNA in the vascular system
- PMID: 38576454
- PMCID: PMC10992703
- DOI: 10.1016/j.omtn.2024.102175
Physiologically based modeling of LNP-mediated delivery of mRNA in the vascular system
Abstract
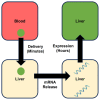
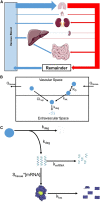
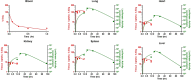
RNA therapeutics are an emerging, powerful class of drugs with potential applications in a wide range of disorders. A central challenge in their development is the lack of clear pharmacokinetic (PK)-pharmacodynamic relationship, in part due to the significant delay between the kinetics of RNA delivery and the onset of pharmacologic response. To bridge this gap, we have developed a physiologically based PK/pharmacodynamic model for systemically administered mRNA-containing lipid nanoparticles (LNPs) in mice. This model accounts for the physiologic determinants of mRNA delivery, active targeting in the vasculature, and differential transgene expression based on nanoparticle coating. The model was able to well-characterize the blood and tissue PKs of LNPs, as well as the kinetics of tissue luciferase expression measured by ex vivo activity in organ homogenates and bioluminescence imaging in intact organs. The predictive capabilities of the model were validated using a formulation targeted to intercellular adhesion molecule-1 and the model predicted nanoparticle delivery and luciferase expression within a 2-fold error for all organs. This modeling platform represents an initial strategy that can be expanded upon and utilized to predict the in vivo behavior of RNA-containing LNPs developed for an array of conditions and across species.
Keywords: MT: Delivery Strategies; drug targeting; lipid nanoparticles; mRNA; nucleic acid delivery; pharmacodynamics; pharmacokinetics; physiologically based pharmacokinetics.
© 2024 The Authors.
Conflict of interest statement
H.P. and D.W. are scientific founders and hold equity in Capstan Therapeutics. S.C.S. and B.L.M. are employees and hold equity in Acuitas Therapeutics. H.P. and D.W. receive research support from BioNTech. H.P., V.V.S., D.W., and V.R.M. are named on patents that describe the use of nucleoside-modified mRNA and targeted LNP.
Figures



References
-
- Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., McClung N., Campos-Outcalt D., Morgan R.L., Mbaeyi S., et al. The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. - DOI - PMC - PubMed
-
- Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., McClung N., Campos-Outcalt D., Morgan R.L., Mbaeyi S., et al. The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Moderna COVID-19 Vaccine - United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;69:1653–1656. doi: 10.15585/mmwr.mm695152e1. - DOI - PMC - PubMed
-
- Parhiz H., Shuvaev V.V., Pardi N., Khoshnejad M., Kiseleva R.Y., Brenner J.S., Uhler T., Tuyishime S., Mui B.L., Tam Y.K., et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Release. 2018;291:106–115. doi: 10.1016/j.jconrel.2018.10.015. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

