Impaired amygdala astrocytic signaling worsens neuropathic pain-associated neuronal functions and behaviors
- PMID: 38576475
- PMCID: PMC10991799
- DOI: 10.3389/fphar.2024.1368634
Impaired amygdala astrocytic signaling worsens neuropathic pain-associated neuronal functions and behaviors
Abstract
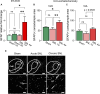
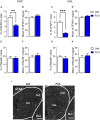
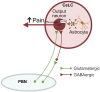
Introduction: Pain is a clinically relevant health care issue with limited therapeutic options, creating the need for new and improved analgesic strategies. The amygdala is a limbic brain region critically involved in the regulation of emotional-affective components of pain and in pain modulation. The central nucleus of amygdala (CeA) serves major output functions and receives nociceptive information via the external lateral parabrachial nucleus (PB). While amygdala neuroplasticity has been linked causally to pain behaviors, non-neuronal pain mechanisms in this region remain to be explored. As an essential part of the neuroimmune system, astrocytes that represent about 40-50% of glia cells within the central nervous system, are required for physiological neuronal functions, but their role in the amygdala remains to be determined for pain conditions. In this study, we measured time-specific astrocyte activation in the CeA in a neuropathic pain model (spinal nerve ligation, SNL) and assessed the effects of astrocyte inhibition on amygdala neuroplasticity and pain-like behaviors in the pain condition. Methods and Results: Glial fibrillary acidic protein (GFAP, astrocytic marker) immunoreactivity and mRNA expression were increased at the chronic (4 weeks post-SNL), but not acute (1 week post-SNL), stage of neuropathic pain. In order to determine the contribution of astrocytes to amygdala pain-mechanisms, we used fluorocitric acid (FCA), a selective inhibitor of astrocyte metabolism. Whole-cell patch-clamp recordings were performed from neurons in the laterocapsular division of the CeA (CeLC) obtained from chronic neuropathic rats. Pre-incubation of brain slices with FCA (100 µM, 1 h), increased excitability through altered hyperpolarization-activated current (Ih) functions, without significantly affecting synaptic responses at the PB-CeLC synapse. Intra-CeA injection of FCA (100 µM) had facilitatory effects on mechanical withdrawal thresholds (von Frey and paw pressure tests) and emotional-affective behaviors (evoked vocalizations), but not on facial grimace score and anxiety-like behaviors (open field test), in chronic neuropathic rats. Selective inhibition of astrocytes by FCA was confirmed with immunohistochemical analyses showing decreased astrocytic GFAP, but not NeuN, signal in the CeA. Discussion: Overall, these results suggest a complex modulation of amygdala pain functions by astrocytes and provide evidence for beneficial functions of astrocytes in CeA in chronic neuropathic pain.
Keywords: amygdala; astrocyte; behavior; electrophysiology; neuroimmune signaling; neuronal excitability; neuropathic pain; neurotransmission.
Copyright © 2024 Mazzitelli, Ponomareva, Presto, John and Neugebauer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
5-HT2C Receptor Knockdown in the Amygdala Inhibits Neuropathic-Pain-Related Plasticity and Behaviors.J Neurosci. 2017 Feb 8;37(6):1378-1393. doi: 10.1523/JNEUROSCI.2468-16.2016. Epub 2016 Dec 23. J Neurosci. 2017. PMID: 28011743 Free PMC article.
-
Small conductance calcium activated potassium (SK) channel dependent and independent effects of riluzole on neuropathic pain-related amygdala activity and behaviors in rats.Neuropharmacology. 2018 Aug;138:219-231. doi: 10.1016/j.neuropharm.2018.06.015. Epub 2018 Jun 13. Neuropharmacology. 2018. PMID: 29908238 Free PMC article.
-
Sex Differences in CGRP Regulation and Function in the Amygdala in a Rat Model of Neuropathic Pain.Front Mol Neurosci. 2022 Jun 3;15:928587. doi: 10.3389/fnmol.2022.928587. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35726298 Free PMC article.
-
Amygdala pain mechanisms.Handb Exp Pharmacol. 2015;227:261-84. doi: 10.1007/978-3-662-46450-2_13. Handb Exp Pharmacol. 2015. PMID: 25846623 Free PMC article. Review.
-
Spinal astrocytes involved in the pathogenesis and treatment of neuropathic pain.Front Cell Neurosci. 2025 Feb 21;19:1547524. doi: 10.3389/fncel.2025.1547524. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40062207 Free PMC article. Review.
Cited by
-
Crosstalk Among Gut Microbiota, Fecal Metabolites, and Amygdala Neuropathology Genes After Ginger Polyphenol Administration in Female Rats with Neuropathic Pain: Evidence for Microbiota-Gut-Brain Connection.Nutrients. 2025 Apr 25;17(9):1444. doi: 10.3390/nu17091444. Nutrients. 2025. PMID: 40362753 Free PMC article.
-
Change in Endogenous Pain Modulation Depending on Emotional States in Healthy Subjects: A Randomized Controlled Trial.Pain Ther. 2024 Oct;13(5):1287-1298. doi: 10.1007/s40122-024-00642-1. Epub 2024 Aug 5. Pain Ther. 2024. PMID: 39102098 Free PMC article.
-
Beneficial Effects of Ginger Root Extract on Pain Behaviors, Inflammation, and Mitochondrial Function in the Colon and Different Brain Regions of Male and Female Neuropathic Rats: A Gut-Brain Axis Study.Nutrients. 2024 Oct 21;16(20):3563. doi: 10.3390/nu16203563. Nutrients. 2024. PMID: 39458557 Free PMC article.
-
Potential common targets of music therapy intervention in neuropsychiatric disorders: the prefrontal cortex-hippocampus -amygdala circuit (a review).Front Hum Neurosci. 2025 Feb 3;19:1471433. doi: 10.3389/fnhum.2025.1471433. eCollection 2025. Front Hum Neurosci. 2025. PMID: 39963392 Free PMC article. Review.
-
Advances in orofacial pain research: a bibliometric analysis.Front Neurol. 2025 Aug 14;16:1609437. doi: 10.3389/fneur.2025.1609437. eCollection 2025. Front Neurol. 2025. PMID: 40895114 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

