Biomechanical Stability of Third-Generation Adjustable Suture Loop Devices Versus Continuous Loop Button Device for Cortical Fixation of ACL Tendon Grafts
- PMID: 38576875
- PMCID: PMC10993678
- DOI: 10.1177/23259671241240375
Biomechanical Stability of Third-Generation Adjustable Suture Loop Devices Versus Continuous Loop Button Device for Cortical Fixation of ACL Tendon Grafts
Abstract
Background: Concerns regarding the primary stability of early adjustable loop button (ALB) devices for cortical fixation of tendon grafts in anterior cruciate ligament reconstruction (ACLR) have led to the development of new implant designs.
Purpose: To evaluate biomechanical stability of recent ALB implants in comparison with a continuous loop button (CLB) device.
Study design: Controlled laboratory study.
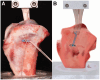
Methods: ACLR was performed in a porcine model (n = 40) using 2-strand porcine flexor tendons with a diameter of 8 mm. Three ALB devices (Infinity Button [ALB1 group]; Tightrope II RT [ALB2 group]; A-TACK [ALB3 group]) and 1 CLB device (FlippTack with polyethylene suture) were used for cortical tendon graft fixation. Cyclic loading (1000 cycles up to 250 N) with complete unloading were applied to the free end of the tendon graft using a uniaxial testing machine, followed by load to failure. Elongation, stiffness, yield load, and ultimate failure load were recorded and compared between the groups using a Kruskal-Wallis test with post hoc Dunn correction.
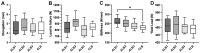
Results: Elongation after 1000 cycles at 250 N was similar between groups (ALB1, 4.5 ± 0.7 mm; ALB2, 4.8 ± 0.8 mm; ALB3, 4.5 ± 0.6 mm; CLB, 4.5 ± 0.8 mm), as was load to failure (ALB1, 838 ± 109 N; ALB2, 930 ± 89 N; ALB3, 809 ± 103 N; CLB, 842 ± 80 N). Stiffness was significantly higher in the ALB1 group compared with the CLB group (262.3 ± 21.6 vs 229.3 ± 15.1 N/mm; P < .05). No significant difference was found between the 4 groups regarding yield load. Constructs failed either by rupture of the loop, breakage of the button, or rupture of the tendon.
Conclusion: The tested third-generation ALB devices for cortical fixation in ACLR withstood cyclic loading with complete unloading without significant differences to a CLB device.
Clinical relevance: The third-generation ALB devices tested in the present study provided biomechanical stability comparable with that of a CLB device. Furthermore, ultimate failure loads of all tested implants exceeded the loads expected to occur in the postoperative period after ACLR.
Keywords: ACL reconstruction; adjustable loop button; continuous loop button; cortical fixation.
© The Author(s) 2024.
Conflict of interest statement
The authors have declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures

Similar articles
-
Biomechanical comparison of 2 anterior cruciate ligament graft preparation techniques for tibial fixation: adjustable-length loop cortical button or interference screw.Am J Sports Med. 2015 Jun;43(6):1380-5. doi: 10.1177/0363546515574062. Epub 2015 Mar 12. Am J Sports Med. 2015. PMID: 25767269
-
Biomechanical Testing of Three Alternative Quadrupled Tendon Graft Constructs With Adjustable Loop Suspensory Fixation for Anterior Cruciate Ligament Reconstruction Compared With Four-Strand Grafts Fixed With Screws and Femoral Fixed Loop Devices.Am J Sports Med. 2019 Mar;47(4):828-836. doi: 10.1177/0363546518825256. Epub 2019 Feb 21. Am J Sports Med. 2019. PMID: 30789779
-
Biomechanical Comparison of 3 Adjustable-Loop Suspensory Devices for All-Inside ACL Reconstruction: A Time-Zero Full-Construct Model.Orthop J Sports Med. 2023 Sep 29;11(9):23259671231201461. doi: 10.1177/23259671231201461. eCollection 2023 Sep. Orthop J Sports Med. 2023. PMID: 37786476 Free PMC article.
-
Fixed- Versus Adjustable-Loop Femoral Cortical Suspension Devices for Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis of Biomechanical Studies.Orthop J Sports Med. 2018 Oct 19;6(10):2325967118801762. doi: 10.1177/2325967118801762. eCollection 2018 Oct. Orthop J Sports Med. 2018. PMID: 30364394 Free PMC article. Review.
-
Is all-inside with suspensory cortical button fixation a superior technique for anterior cruciate ligament reconstruction surgery? A systematic review and meta-analysis.BMC Musculoskelet Disord. 2020 Jul 7;21(1):445. doi: 10.1186/s12891-020-03471-3. BMC Musculoskelet Disord. 2020. PMID: 32635920 Free PMC article.
Cited by
-
Knotless Suture Anchors Display Favorable Elongation but an Inferior Ultimate Failure Load Versus Titanium Suture Anchors and All-Suture Anchors: A Biomechanical Comparison in a Porcine Model.Orthop J Sports Med. 2024 Dec 13;12(12):23259671241300520. doi: 10.1177/23259671241300520. eCollection 2024 Dec. Orthop J Sports Med. 2024. PMID: 39678438 Free PMC article.
References
-
- Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663-670. - PubMed
-
- Ahmad CS, Gardner TR, Groh M, Arnouk J, Levine WN. Mechanical properties of soft tissue femoral fixation devices for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):635-640. - PubMed
-
- Ahmad SS, Hirschmann MT, Voumard B, et al.. Adjustable loop ACL suspension devices demonstrate less reliability in terms of reproducibility and irreversible displacement. Knee Surg Sports Traumatol Arthrosc. 2018;26(5):1392-1398. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

