The roles of non-coding RNAs in Hirschsprung's disease
- PMID: 38577013
- PMCID: PMC10990754
- DOI: 10.1016/j.ncrna.2024.02.015
The roles of non-coding RNAs in Hirschsprung's disease
Abstract
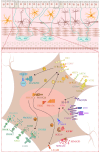
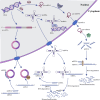
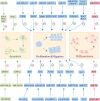
Hirschsprung's disease (HSCR) is a congenital disorder characterized by the absence of ganglion cells in the colon, leading to various intestinal complications. The etiology of HSCR stems from complex genetic and environmental interactions, of which the intricate roles of non-coding RNAs (ncRNAs) are a key area of research. However, the roles of ncRNAs in the pathogenesis of HSCR have not been fully elucidated. In order to understand the variety of symptoms caused by HSCR and develop new therapeutic approaches, it is essential to understand the underlying biological genetic basis of HSCR. This review presents a comprehensive overview of the current understanding regarding the involvement of ncRNAs in HSCR, including microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs). Additionally, it provides a summary of the molecular mechanisms through which ncRNAs regulate the expression of genes related to the proliferation, migration, and differentiation of intestinal neural crest cells, thereby contributing to the advancement of HSCR research.
Keywords: CircRNAs; Hirschsprung's disease; LncRNAs; MiRNAs; NcRNAs.
© 2024 The Authors.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

