Early establishment of chloride homeostasis in CRH neurons is altered by prenatal stress leading to fetal HPA axis dysregulation
- PMID: 38577026
- PMCID: PMC10994000
- DOI: 10.3389/fnmol.2024.1373337
Early establishment of chloride homeostasis in CRH neurons is altered by prenatal stress leading to fetal HPA axis dysregulation
Abstract
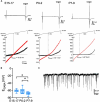
Corticotropin-releasing hormone (CRH) neurons play an important role in the regulation of neuroendocrine responses to stress. The excitability of CRH neurons is regulated by inhibitory GABAergic inputs. However, it is unclear when GABAergic regulation of CRH neurons is established during fetal brain development. Furthermore, the exact progression of the developmental shift of GABA action from depolarization to hyperpolarization remains unelucidated. Considering the importance of CRH neuron function in subsequent hypothalamic-pituitary-adrenal (HPA) axis regulation during this critical phase of development, we investigated the ontogeny of GABAergic inputs to CRH neurons and consequent development of chloride homeostasis. Both CRH neuron soma in the paraventricular nucleus (PVN) and axons projecting to the median eminence could be identified at embryonic day 15 (E15). Using acute slices containing the PVN of CRF-VenusΔNeo mice, gramicidin perforated-patch clamp-recordings of CRH neurons at E15, postnatal day 0 (P0), and P7 were performed to evaluate the developmental shift of GABA action. The equilibrium potential of GABA (EGABA) was similar between E15 and P0 and showed a further hyperpolarizing shift between P0 and P7 that was comparable to EGABA values in adult CRH neurons. GABA primarily acted as an inhibitory signal at E15 and KCC2 expression was detected in CRH neurons at this age. Activation of the HPA axis has been proposed as the primary mechanism through which prenatal maternal stress shapes fetal development and subsequent long-term disease risk. We therefore examined the impact of maternal food restriction stress on the development of chloride homeostasis in CRH neurons. We observed a depolarization shift of EGABA in CRH neurons of pups exposed to maternal food restriction stress. These results suggest that Cl- homeostasis in early developmental CRH neurons attains mature intracellular Cl- levels, GABA acts primarily as inhibitory, and CRH neurons mature and function early compared with neurons in other brain regions, such as the cortex and hippocampus. Maternal food restriction stress alters chloride homeostasis in CRH neurons of pups, reducing their inhibitory control by GABA. This may contribute to increased CRH neuron activity and cause activation of the HPA axis in pups.
Keywords: CRH neuron; GABA; HPA axis; KCC2; chloride homeostasis; prenatal stress.
Copyright © 2024 Watanabe, Sinha, Shinmyo and Fukuda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Chronic Unpredictable Mild Stress Induces Loss of GABA Inhibition in Corticotrophin-Releasing Hormone-Expressing Neurons through NKCC1 Upregulation.Neuroendocrinology. 2017;104(2):194-208. doi: 10.1159/000446114. Epub 2016 Apr 15. Neuroendocrinology. 2017. PMID: 27077366 Free PMC article.
-
Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice.Psychoneuroendocrinology. 2018 Apr;90:182-193. doi: 10.1016/j.psyneuen.2017.12.003. Epub 2017 Dec 21. Psychoneuroendocrinology. 2018. PMID: 29274662 Free PMC article.
-
A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence.Sci Adv. 2016 Aug 17;2(8):e1501723. doi: 10.1126/sciadv.1501723. eCollection 2016 Aug. Sci Adv. 2016. PMID: 27540587 Free PMC article.
-
The stress system in the human brain in depression and neurodegeneration.Ageing Res Rev. 2005 May;4(2):141-94. doi: 10.1016/j.arr.2005.03.003. Ageing Res Rev. 2005. PMID: 15996533 Review.
-
Heterosynaptic modulation in the paraventricular nucleus of the hypothalamus.Neuropharmacology. 2019 Aug;154:87-95. doi: 10.1016/j.neuropharm.2018.11.004. Epub 2018 Nov 5. Neuropharmacology. 2019. PMID: 30408488 Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

