Etanercept-synthesizing adipose-derived stem cell secretome: A promising therapeutic option for inflammatory bowel disease
- PMID: 38577094
- PMCID: PMC10989350
- DOI: 10.4240/wjgs.v16.i3.882
Etanercept-synthesizing adipose-derived stem cell secretome: A promising therapeutic option for inflammatory bowel disease
Abstract
Background: Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract, with tumor necrosis factor (TNF)-α playing a key role in its pathogenesis. Etanercept, a decoy receptor for TNF, is used to treat inflammatory conditions. The secretome derived from adipose-derived stem cells (ASCs) has anti-inflammatory effects, making it a promising therapeutic option for IBD.
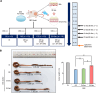
Aim: To investigate the anti-inflammatory effects of the secretome obtained from ASCs synthesizing etanercept on colon cells and in a dextran sulfate sodium (DSS)-induced IBD mouse model.
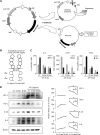
Methods: ASCs were transfected with etanercept-encoding mini-circle plasmids to create etanercept-producing cells. The secretory material from these cells was then tested for anti-inflammatory effects both in vitro and in a DSS-induced IBD mouse model.
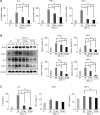
Results: This study revealed promising results indicating that the group treated with the secretome derived from etanercept-synthesizing ASCs [Etanercept-Secretome (Et-Sec) group] had significantly lower expression levels of inflammatory mediators, such as interleukin-6, Monocyte Chemoattractant Protein-1, and TNF-α, when compared to the control secretome (Ct-Sec). Moreover, the Et-Sec group exhibited a marked therapeutic effect in terms of preserving the architecture of intestinal tissue compared to the Ct-Sec.
Conclusion: These results suggest that the secretome derived from ASCs that synthesize etanercept has potential as a therapeutic agent for the treatment of IBD, potentially enhancing treatment efficacy by merging the anti-inflammatory qualities of the ASC secretome with etanercept's targeted approach to better address the multifaceted pathophysiology of IBD.
Keywords: Adipose-derived stem cells; Etanercept; Inflammatory bowel disease; Secretome; Tumor necrosis factor-α.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Dr. Lee reports grants from National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), during the conduct of the study; In addition, Dr. Lee has a patent 10-2023-0193643 pending to Catholic University Industry-Academic Cooperation Foundation.
Figures


Similar articles
-
A Novel Hepatic Anti-Fibrotic Strategy Utilizing the Secretome Released from Etanercept-Synthesizing Adipose-Derived Stem Cells.Int J Mol Sci. 2019 Dec 13;20(24):6302. doi: 10.3390/ijms20246302. Int J Mol Sci. 2019. PMID: 31847135 Free PMC article.
-
Conditioned secretome of adipose-derived stem cells improves dextran sulfate sodium-induced colitis in mice.World J Gastroenterol. 2021 Jun 21;27(23):3342-3356. doi: 10.3748/wjg.v27.i23.3342. World J Gastroenterol. 2021. PMID: 34163116 Free PMC article.
-
Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling.Stem Cells Transl Med. 2016 Jun;5(6):816-25. doi: 10.5966/sctm.2015-0191. Epub 2016 Apr 21. Stem Cells Transl Med. 2016. PMID: 27102647 Free PMC article.
-
Inflammatory bowel disease as a paradoxical effect of anti-TNF alpha therapy.Gastroenterol Hepatol. 2017 Feb;40(2):117-121. doi: 10.1016/j.gastrohep.2016.01.011. Epub 2016 Mar 15. Gastroenterol Hepatol. 2017. PMID: 26993096 Review. English, Spanish.
-
Adipose-Derived Stem Cells Secretome and Its Potential Application in "Stem Cell-Free Therapy".Biomolecules. 2021 Jun 13;11(6):878. doi: 10.3390/biom11060878. Biomolecules. 2021. PMID: 34199330 Free PMC article. Review.
References
-
- Colombini A, Libonati F, Lopa S, Ragni E, De Luca P, Zagra L, Sinigaglia F, Moretti M, de Girolamo L. Immunomodulatory potential of secretome from cartilage cells and mesenchymal stromal cells in an arthritic context: From predictive fiction toward reality. Front Med (Lausanne) 2022;9:992386. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

