Exploring the impacts of ketogenic diet on reversible hepatic steatosis: initial analysis in male mice
- PMID: 38577162
- PMCID: PMC10991688
- DOI: 10.3389/fnut.2024.1290540
Exploring the impacts of ketogenic diet on reversible hepatic steatosis: initial analysis in male mice
Abstract
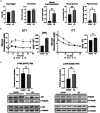
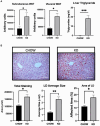
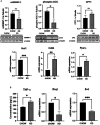
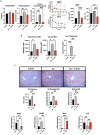
Metabolic dysfunction-associated fatty liver disease (MAFLD) is the most common chronic liver disease. Ketogenic diet (KD), a diet with very low intake in carbohydrates, gained popularity as a weight-loss approach. However, in mice models, it has been reported that an excess exposition of dietary fat induces hepatic insulin resistance and steatosis. However, data published is inconsistent. Herein, we investigated in a mouse model, the metabolic effects of KD and its contribution to the pathogenesis of NALFD. Mice were exposed to KD or CHOW diet for 12 weeks while a third group was exposed to KD for also 12 weeks and then switched to CHOW diet for 4 weeks to determine if we can rescue the phenotype. We evaluated the effects of diet treatments on fat distribution, glucose, and insulin homeostasis as well as hepatic steatosis. Mice fed with KD developed glucose intolerance but not insulin resistance accompanied by an increase of inflammation. KD-fed mice showed an increase of fat accumulation in white adipose tissue and liver. This effect could be explained by an increase in fat uptake by the liver with no changes of catabolism leading to MAFLD. Interestingly, we were able to rescue the phenotype by switching KD-fed mice for 4 weeks on a CHOW diet. Our studies demonstrate that even if mice develop hepatic steatosis and glucose intolerance after 12 weeks of KD, they do not develop insulin resistance and more importantly, the phenotype can be reversed by switching the mice from a KD to a CHOW.
Keywords: glucose intolerance; hepatic steatosis; inflammation; insulin resistance; ketogenic diet; rescue.
Copyright © 2024 Ravaut, Carneiro and Mounier.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A low-carbohydrate diet induces hepatic insulin resistance and metabolic associated fatty liver disease in mice.Mol Metab. 2023 Mar;69:101675. doi: 10.1016/j.molmet.2023.101675. Epub 2023 Jan 19. Mol Metab. 2023. PMID: 36682412 Free PMC article.
-
Short-term feeding of a ketogenic diet induces more severe hepatic insulin resistance than an obesogenic high-fat diet.J Physiol. 2018 Oct;596(19):4597-4609. doi: 10.1113/JP275173. Epub 2018 Aug 8. J Physiol. 2018. PMID: 30089335 Free PMC article.
-
Long-term ketogenic diet contributes to glycemic control but promotes lipid accumulation and hepatic steatosis in type 2 diabetic mice.Nutr Res. 2016 Apr;36(4):349-358. doi: 10.1016/j.nutres.2015.12.002. Epub 2015 Dec 7. Nutr Res. 2016. PMID: 27001280
-
The impact of ketogenic diet on some metabolic and non-metabolic diseases: Evidence from human and animal model experiments.Food Sci Nutr. 2024 Jan 8;12(3):1444-1464. doi: 10.1002/fsn3.3873. eCollection 2024 Mar. Food Sci Nutr. 2024. PMID: 38455178 Free PMC article. Review.
-
Low-Carbohydrate High-Fat Diet: A SWOC Analysis.Metabolites. 2022 Nov 17;12(11):1126. doi: 10.3390/metabo12111126. Metabolites. 2022. PMID: 36422267 Free PMC article. Review.
Cited by
-
Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs.Int J Mol Sci. 2024 Jun 27;25(13):7076. doi: 10.3390/ijms25137076. Int J Mol Sci. 2024. PMID: 39000187 Free PMC article. Review.
-
Diagnostic indicators and lifestyle interventions of metabolic-associated fatty liver disease.Front Nutr. 2024 Jun 14;11:1424246. doi: 10.3389/fnut.2024.1424246. eCollection 2024. Front Nutr. 2024. PMID: 38946789 Free PMC article. Review.
-
Sex- and Age-Specific Differences in Mice Fed a Ketogenic Diet.Nutrients. 2024 Aug 16;16(16):2731. doi: 10.3390/nu16162731. Nutrients. 2024. PMID: 39203867 Free PMC article.
-
Butyrate improves testicular spermatogenic dysfunction induced by a high-fat diet.Transl Androl Urol. 2025 Mar 30;14(3):627-636. doi: 10.21037/tau-2024-660. Epub 2025 Mar 26. Transl Androl Urol. 2025. PMID: 40226081 Free PMC article.
-
Targeting ketone body metabolism to treat fatty liver disease.J Pharm Pharm Sci. 2024 Sep 30;27:13375. doi: 10.3389/jpps.2024.13375. eCollection 2024. J Pharm Pharm Sci. 2024. PMID: 39403635 Free PMC article. Review.
References
-
- Hallberg SJ, Mckenzie AL, Williams PT, Bhanpuri NH, Peters AL, Campbell WW, et al. . Author correction: effectiveness and safety of a novel care model for the management of Type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. (2018) 9:613–21. doi: 10.1007/s13300-018-0386-4, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

