PRaG 3.0 therapy for human epidermal growth factor receptor 2-positive metastatic pancreatic ductal adenocarcinoma: A case report
- PMID: 38577174
- PMCID: PMC10989490
- DOI: 10.3748/wjg.v30.i9.1237
PRaG 3.0 therapy for human epidermal growth factor receptor 2-positive metastatic pancreatic ductal adenocarcinoma: A case report
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is a highly fatal disease with limited effective treatment especially after first-line chemotherapy. The human epidermal growth factor receptor 2 (HER-2) immunohistochemistry (IHC) positive is associated with more aggressive clinical behavior and shorter overall survival in PDAC.
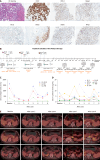
Case summary: We present a case of multiple metastatic PDAC with IHC mismatch repair proficient but HER-2 IHC weakly positive at diagnosis that didn't have tumor regression after first-line nab-paclitaxel plus gemcitabine and PD-1 inhibitor treatment. A novel combination therapy PRaG 3.0 of RC48 (HER2-antibody-drug conjugate), radiotherapy, PD-1 inhibitor, granulocyte-macrophage colony-stimulating factor and interleukin-2 was then applied as second-line therapy and the patient had confirmed good partial response with progress-free-survival of 6.5 months and overall survival of 14.2 month. She had not developed any grade 2 or above treatment-related adverse events at any point. Percentage of peripheral CD8+Temra and CD4+Temra were increased during first two activation cycles of PRaG 3.0 treatment containing radiotherapy but deceased to the baseline during the maintenance cycles containing no radiotherapy.
Conclusion: PRaG 3.0 might be a novel strategy for HER2-positive metastatic PDAC patients who failed from previous first-line approach and even PD-1 immunotherapy but needs more data in prospective trials.
Keywords: Case report; Human epidermal growth factor receptor 2; Novel combination therapy; PRaG 3.0 therapy; Pancreatic ductal adenocarcinoma.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Authors declare that there were no competing interests.
Figures
References
-
- Wu J, Cai J. Dilemma and Challenge of Immunotherapy for Pancreatic Cancer. Dig Dis Sci. 2021;66:359–368. - PubMed
-
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

