Phase 1, first-in-human study of TYRP1-TCB (RO7293583), a novel TYRP1-targeting CD3 T-cell engager, in metastatic melanoma: active drug monitoring to assess the impact of immune response on drug exposure
- PMID: 38577337
- PMCID: PMC10991832
- DOI: 10.3389/fonc.2024.1346502
Phase 1, first-in-human study of TYRP1-TCB (RO7293583), a novel TYRP1-targeting CD3 T-cell engager, in metastatic melanoma: active drug monitoring to assess the impact of immune response on drug exposure
Abstract
Introduction: Although checkpoint inhibitors (CPIs) have improved outcomes for patients with metastatic melanoma, those progressing on CPIs have limited therapeutic options. To address this unmet need and overcome CPI resistance mechanisms, novel immunotherapies, such as T-cell engaging agents, are being developed. The use of these agents has sometimes been limited by the immune response mounted against them in the form of anti-drug antibodies (ADAs), which is challenging to predict preclinically and can lead to neutralization of the drug and loss of efficacy.
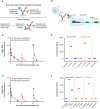
Methods: TYRP1-TCB (RO7293583; RG6232) is a T-cell engaging bispecific (TCB) antibody that targets tyrosinase-related protein 1 (TYRP1), which is expressed in many melanomas, thereby directing T cells to kill TYRP1-expressing tumor cells. Preclinical studies show TYRP1-TCB to have potent anti-tumor activity. This first-in-human (FIH) phase 1 dose-escalation study characterized the safety, tolerability, maximum tolerated dose/optimal biological dose, and pharmacokinetics (PK) of TYRP1-TCB in patients with metastatic melanoma (NCT04551352).
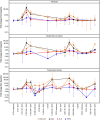
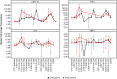
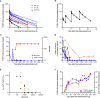
Results: Twenty participants with cutaneous, uveal, or mucosal TYRP1-positive melanoma received TYRP1-TCB in escalating doses (0.045 to 0.4 mg). All participants experienced ≥1 treatment-related adverse event (TRAE); two participants experienced grade 3 TRAEs. The most common toxicities were grade 1-2 cytokine release syndrome (CRS) and rash. Fractionated dosing mitigated CRS and was associated with lower levels of interleukin-6 and tumor necrosis factor-alpha. Measurement of active drug (dual TYPR1- and CD3-binding) PK rapidly identified loss of active drug exposure in all participants treated with 0.4 mg in a flat dosing schedule for ≥3 cycles. Loss of exposure was associated with development of ADAs towards both the TYRP1 and CD3 domains. A total drug PK assay, measuring free and ADA-bound forms, demonstrated that TYRP1-TCB-ADA immune complexes were present in participant samples, but showed no drug activity in vitro.
Discussion: This study provides important insights into how the use of active drug PK assays, coupled with mechanistic follow-up, can inform and enable ongoing benefit/risk assessment for individuals participating in FIH dose-escalation trials. Translational studies that lead to a better understanding of the underlying biology of cognate T- and B-cell interactions, ultimately resulting in ADA development to novel biotherapeutics, are needed.
Keywords: ADA; TCB; TYRP1; anti-drug antibody; antibody; bispecific; immunogenicity; metastatic melanoma.
Copyright © 2024 Spreafico, Couselo, Irmisch, Bessa, Au-Yeung, Bechter, Svane, Sanmamed, Gambardella, McKean, Callahan, Dummer, Klein, Umaña, Justies, Heil, Fahrni, Opolka-Hoffmann, Waldhauer, Bleul, Staack, Karanikas and Fowler.
Conflict of interest statement
Authors AI, JB, CK, PU, NJ, FH, LF, EO-H, IW, CB, RFS, VK and SF are or were employees of F. Hoffmann-La Roche Ltd. Author AS discloses employment Princess Margaret Cancer Centre, University Health Network, research funding Alkermes, ALX Oncology, Amgen, Array Biopharma/Pfizer, AstraZeneca/Medimmune, Bayer, BMS, Genentech, GSK, Janssen Oncology/Johnson & Johnson, Merck, Northern Biologics, Novartis, Nubiyota, Oncorus, Roche, Regeneron, Seagen, Servier, Surface Oncology, Symphogen, Treadwell, and advisory committees BMS, Merck. Author EM discloses honoraria BMS, MSD, Novartis, Pierre Fabre, Sanofi and advisory committees and speaker’s bureau BMS, MSD, Novartis, Pierre Fabre, Sanofi. Author GA-Y discloses research funding AstraZeneca, Roche/Genentech. Author OB discloses advisory committees BMS, MSD, Pierre Fabre, Sanofi. Author IS discloses consultancy IO Biotech, MSD, Novartis, Pierre Fabre, TILT Biotherapeutics, research funding Adaptimmune, Asgard Biotech, Enara Bio, Evaxion Biotech, IO Biotech, Lytix Biopharma, TILT Biotherapeutics, honoraria BMS, MSD, Novartis, Novo Nordisk, Pierre Fabre, Sanofi Aventis, Takeda, patents and royalties IO Biotech, and investigator for initiated clinical trial BMS. Author MS discloses consultancy BMS, Numab, research funding Roche, advisory committees Numab, and speaker’s bureau BMS, MSD. Author VG discloses research funding Bayer, Boehringer, Roche and institutional funding Amcure, Astelas, Astra Zeneca, Bayer, BeiGene, BMS, FibroGen, Genentech, Lilly, Medimmune, Merck Serono, MSD, Natera, Novartis, Roche, Servier, Sierra Oncology, Takeda. Author MM discloses consultancy Castle Biosciences, Eisai, IQVIA, Merck, Moderna, Pfizer and research funding Aadi Biosciences, Alpine Immune Sciences, Arcus Biosciences, Arvinas, Ascentage Pharma Group, ASCO, Astellas, Aulos Bioscience, Bayer, Bicycle Therapeutics, Biomed Valley Discoveries, BioNTech, BMS, C4 Therapeutics, Dragonfly Therapeutics, EMD Serono, Epizyme, Erasca, Exelixis, Foghorn Therapeutics, G1 Therapeutics, Genentech/Roche, Gilead Sciences, GSK, IDEAYA Biosciences, Ikena Oncology, ImmVira Pharma, Infinity Pharmaceuticals, Jacobio Pharmaceuticals, Kechow Pharma, Kezar Life Sciences, Kinnate BioPharma, MedImmune, Mereo BioPharma, Metabomed, Moderna, NBE Therapeutics, Nektar, Novartis, NucMito Pharmaceuticals, OncoC4, Oncorus, OnKure, PACT Pharma, Pfizer, Plexxikon, Poseida, Prelude Therapeutics, Pyramid Biosciences, Regeneron, Sapience Therapeutics, Scholar Rock, Seattle Genetics, Synthrox, Tenebio, Tempest Therapeutics, Tizona Therapeutics, TMUNITY Therapeutics, TopAlliance Biosciences, Xilio. Author MC discloses consulting and advisory boards BMS, AstraZeneca, Immunocore, Regeneron, Merck. Author RD discloses consulting and/or advisory relationships Alligator, Amgen, Axivox, BMS, Catalym, MSD, Novartis, Pfizer, Pierre Fabre, Regeneron, Roche, Simcere, Sanofi, Second Genome, Sun Pharma, Takeda, TouchIME, T3 Pharma. Authors CK, LF, IW, and VK disclose employment, equity, and patents and royalties Roche. Authors NJ and EO-H disclose employment Roche. Authors JB, FH, CB, RS, PU, AI and SF discloses employment and equity Roche. The Reviewer FD declared a past co-authorship with the author RD to the handling editor. The authors declare that this study received funding from F. Hoffman-La Roche Ltd. F. Hoffmann-La Roche Ltd was involved in the study design, collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
Figures
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

