Sunlight-driven and gram-scale vanillin production via Mn-defected γ-MnO2 catalyst in aqueous environment
- PMID: 38577364
- PMCID: PMC10988585
- DOI: 10.1039/d3sc05654f
Sunlight-driven and gram-scale vanillin production via Mn-defected γ-MnO2 catalyst in aqueous environment
Abstract
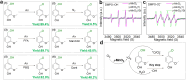
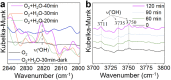
The production of vanillin from biomass offers a sustainable route for synthesizing daily-use chemicals. However, achieving sunlight-driven vanillin synthesis through H2O activation in an aqueous environment poses challenges due to the high barrier of H2O dissociation. In this study, we have successfully developed an efficient approach for gram-scale vanillin synthesis in an aqueous reaction, employing Mn-defected γ-MnO2 as a photocatalyst at room temperature. Density functional theory calculations reveal that the presence of defective Mn species (Mn3+) significantly enhances the adsorption of vanillyl alcohol and H2O onto the surface of the γ-MnO2 catalyst. Hydroxyl radical (˙OH) species are formed through H2O activation with the assistance of sunlight, playing a pivotal role as oxygen-reactive species in the oxidation of vanillyl alcohol into vanillin. The Mn-defected γ-MnO2 catalyst exhibits exceptional performance, achieving up to 93.4% conversion of vanillyl alcohol and 95.7% selectivity of vanillin under sunlight. Notably, even in a laboratory setting during the daytime, the Mn-defected γ-MnO2 catalyst demonstrates significantly higher catalytic performance compared to the dark environment. This work presents a highly effective and promising strategy for low-cost and environmentally benign vanillin synthesis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
NiS/Cd0.6 Zn0.4 S Schottky Junction Bifunctional Photocatalyst for Sunlight-Driven Highly Selective Catalytic Oxidation of Vanillyl Alcohol Towards Vanillin Coupled with Hydrogen Evolution Reaction.Small. 2023 Sep;19(39):e2302330. doi: 10.1002/smll.202302330. Epub 2023 May 31. Small. 2023. PMID: 37259262
-
Synthesis of palladium-decorated defective tungsten oxide heterostructures with enhanced photothermal catalytic activity for hydrodeoxygenation of vanillin.J Colloid Interface Sci. 2024 Oct 15;672:520-532. doi: 10.1016/j.jcis.2024.05.119. Epub 2024 May 16. J Colloid Interface Sci. 2024. PMID: 38839513
-
Metabolic engineering of hairy root cultures in Beta vulgaris for enhanced production of vanillin, 4-hydroxybenzoic acid, and vanillyl alcohol.Front Bioeng Biotechnol. 2024 Oct 2;12:1435190. doi: 10.3389/fbioe.2024.1435190. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39416280 Free PMC article.
-
Water-Driven Surface Lattice Oxygen Activation in MnO2 for Promoted Low-Temperature NH3-SCR.Environ Sci Technol. 2024 Sep 9. doi: 10.1021/acs.est.4c06313. Online ahead of print. Environ Sci Technol. 2024. PMID: 39250812
-
Synthesis of α-MnO2-like rod catalyst using YMn2O5 A-site sacrificial strategy for efficient benzene oxidation.J Hazard Mater. 2021 Feb 5;403:123811. doi: 10.1016/j.jhazmat.2020.123811. Epub 2020 Sep 14. J Hazard Mater. 2021. PMID: 33264910
References
-
- Schaefer B., Natural Products in the Chemical Industry, Springer, Berlin, 2014
-
- Zhu D. Xu L. Sethupathy S. Si H. Ahmad F. Zhang R. Zhang W. Yang B. Sun J. Green Chem. 2021;23:9554–9570. doi: 10.1039/D1GC02692E. - DOI
LinkOut - more resources
Full Text Sources

