Fe3O4@SiO2 core/shell functionalized by gallic acid: a novel, robust, and water-compatible heterogeneous magnetic nanocatalyst for environmentally friendly synthesis of acridine-1,8-diones
- PMID: 38577428
- PMCID: PMC10990003
- DOI: 10.1039/d4ra00629a
Fe3O4@SiO2 core/shell functionalized by gallic acid: a novel, robust, and water-compatible heterogeneous magnetic nanocatalyst for environmentally friendly synthesis of acridine-1,8-diones
Abstract
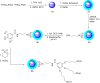
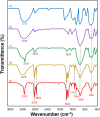
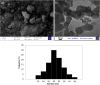
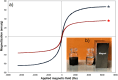
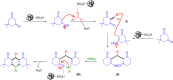
In this study, we conveniently prepared a novel robust heterogeneous magnetic nanocatalyst using a Fe3O4@SiO2 core/shell stabilized by gallic acid. The catalyst was completely characterized by various physicochemical techniques, including infrared spectroscopy (FT-IR), X-ray diffraction (XRD), dynamic light scattering (DLS), transmission electron microscopy (TEM), field emission scanning electron microscopy (FE-SEM), thermogravimetric analysis (TGA), potentiometric titration, energy dispersive X-ray microanalysis (EDX), vibrating sample magnetometer (VSM), zeta potential analysis, and BET. The potential ability of the newly developed sulfonated nanocatalyst was then exploited in the multicomponent synthesis of acridine-1,8-dione derivatives by considering the green chemistry matrix and under mild conditions. Various aldehydes and amines were smoothly reacted with dimedone, affording the desired products in good to excellent yields. The introduction of sulfonic groups using gallic acid allowed the development of a water-compatible and highly recyclable catalytic system for reactions in an aqueous environment. The prepared catalyst can be readily magnetically separated and reused eight times without significant loss of activity. High synthetic efficiency, using a recyclable and eco-sustainable catalyst under mild conditions, and easy product isolation are salient features of this catalytic system, which makes this protocol compatible with the demands of green chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






Similar articles
-
Diphenyl diselenide immobilized on magnetic nanoparticles: A novel and retrievable heterogeneous catalyst in the oxidation of aldehydes under mild and green conditions.J Colloid Interface Sci. 2018 Jan 1;509:485-494. doi: 10.1016/j.jcis.2017.09.034. Epub 2017 Sep 8. J Colloid Interface Sci. 2018. PMID: 28923746
-
Design, Preparation and Characterization of MoO3H-functionalized Fe3O4@SiO2 Magnetic Nanocatalyst and Application for the One-pot Multicomponent Reactions.Acta Chim Slov. 2017 Sep;64(3):707-713. doi: 10.17344/acsi.2017.3208. Acta Chim Slov. 2017. PMID: 28862294
-
Mo@GAA-Fe3O4 MNPs: a highly efficient and environmentally friendly heterogeneous magnetic nanocatalyst for the synthesis of polyhydroquinoline derivatives.RSC Adv. 2021 Mar 11;11(18):10497-10511. doi: 10.1039/d1ra00396h. eCollection 2021 Mar 10. RSC Adv. 2021. PMID: 35423550 Free PMC article.
-
Magnetite Nanoparticles-Supported APTES as a Powerful and Recoverable Nanocatalyst for the Preparation of 2-Amino-5,10-dihydro- 5,10-dioxo-4H-benzo[g]chromenes and Tetrahydrobenzo[g]quinoline-5,10- diones.Comb Chem High Throughput Screen. 2017;20(1):64-76. doi: 10.2174/1386207319666161223121612. Comb Chem High Throughput Screen. 2017. PMID: 28017132
-
Preparation of core/shell nanostructure Fe(3)O(4)@PEG400-SO(3)H as heterogeneous and magnetically recyclable nanocatalyst for one-pot synthesis of substituted pyrroles by Paal-Knorr reaction at room temperature.J Colloid Interface Sci. 2017 Jun 15;496:177-187. doi: 10.1016/j.jcis.2017.02.023. Epub 2017 Feb 13. J Colloid Interface Sci. 2017. PMID: 28219034
References
-
- Ganapathe L. S. Mohamed M. A. Mohamad Yunus R. Berhanuddin D. D. Magnetochemistry. 2020;6:68.
-
- Dee R. H. Proc. IEEE. 2008;96:1775–1785.
-
- Park C. P. Kim D.-P. Angew. Chem., Int. Ed. 2010;49:6825–6829. - PubMed
-
- Wicki A. Witzigmann D. Balasubramanian V. Huwyler J. J. Controlled Release. 2015;200:138–157. - PubMed
- Cai X. Zhu Q. Zeng Y. Zeng Q. Chen X. Zhan Y. Int. J. Nanomed. 2019:8321–8344. - PMC - PubMed
- Ulbrich K. Hola K. Subr V. Bakandritsos A. Tucek J. Zboril R. Chem. Rev. 2016;116:5338–5431. - PubMed
- Karade V. Sharma A. Dhavale R. Dhavale R. Shingte S. Patil P. Kim J. Zahn D. Chougale A. Salvan G. Sci. Rep. 2021;11:5674. - PMC - PubMed
-
- Zahmatkesh S. Esmaeilpour M. Javidi J. RSC Adv. 2016;6:90154–90164.
- Polshettiwar V. Luque R. Fihri A. Zhu H. Bouhrara M. Basset J.-M. Chem. Rev. 2011;111:3036–3075. - PubMed
- Javidi J. Esmaeilpour M. Mater. Res. Bull. 2016;73:409–422.
LinkOut - more resources
Full Text Sources

