Predictive factors and the management of hyperglycemia in patients with acromegaly and Cushing's disease receiving pasireotide treatment: post hoc analyses from the SOM230B2219 study
- PMID: 38577574
- PMCID: PMC10993249
- DOI: 10.3389/fendo.2024.1250822
Predictive factors and the management of hyperglycemia in patients with acromegaly and Cushing's disease receiving pasireotide treatment: post hoc analyses from the SOM230B2219 study
Abstract
Introduction: Pasireotide, a somatostatin receptor ligand, is approved for treating acromegaly and Cushing's disease (CD). Hyperglycemia during treatment can occur because of the drug's mechanism of action, although treatment discontinuation is rarely required. The prospective, randomized, Phase IV SOM230B2219 (NCT02060383) trial was designed to assess optimal management of pasireotide-associated hyperglycemia. Here, we investigated predictive factors for requiring antihyperglycemic medication during pasireotide treatment.
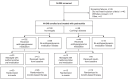
Methods: Participants with acromegaly or CD initiated long-acting pasireotide 40 mg/28 days intramuscularly (acromegaly) or pasireotide 600 μg subcutaneously twice daily during pre-randomization (≤16 weeks). Those who did not need antihyperglycemic medication, were managed with metformin, or received insulin from baseline entered an observational arm ending at 16 weeks. Those who required additional/alternative antihyperglycemic medication to metformin were randomized to incretin-based therapy or insulin for an additional 16 weeks. Logistic-regression analyses evaluated quantitative and qualitative factors for requiring antihyperglycemic medication during pre-randomization.
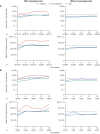
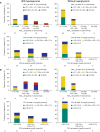
Results: Of 190 participants with acromegaly and 59 with CD, 88 and 15, respectively, did not need antihyperglycemic medication; most were aged <40 years (acromegaly 62.5%, CD 86.7%), with baseline glycated hemoglobin (HbA1c) <6.5% (<48 mmol/mol; acromegaly 98.9%, CD 100%) and fasting plasma glucose (FPG) <100 mg/dL (<5.6 mmol/L; acromegaly 76.1%, CD 100%). By logistic regression, increasing baseline HbA1c (odds ratio [OR] 3.6; P=0.0162) and FPG (OR 1.0; P=0.0472) and history of diabetes/pre-diabetes (OR 3.0; P=0.0221) predicted receipt of antihyperglycemic medication in acromegaly participants; increasing baseline HbA1c (OR 12.6; P=0.0276) was also predictive in CD participants. Investigator-reported hyperglycemia-related adverse events were recorded in 47.9% and 54.2% of acromegaly and CD participants, respectively, mainly those with diabetes/pre-diabetes.
Conclusion: Increasing age, HbA1c, and FPG and pre-diabetes/diabetes were associated with increased likelihood of requiring antihyperglycemic medication during pasireotide treatment. These risk factors may be used to identify those who need more vigilant monitoring to optimize outcomes during pasireotide treatment.
Keywords: Cushing’s disease; acromegaly; diabetes mellitus; glucose intolerance; hyperglycemia; pasireotide; pituitary adenoma.
Copyright © 2024 Feldt-Rasmussen, Bolanowski, Zhang, Yu, Witek, Kalra, Kietsiriroje, Piacentini, Pedroncelli and Samson.
Conflict of interest statement
UF-R reports travel grants and speaker honoraria from Ipsen, Recordati, and Novo Nordisk and advisory board fees from Novartis, Recordati, Novo Nordisk, and Xeris Pharmaceuticals Strongbridge. UF-R’s research salary was sponsored by a grant from Kirsten and Freddy Johansen’s Fund. MB reports receiving travel grants and speaker fees from Amryt, Recordati, Pfizer, Novartis, Ipsen, IBSA, Teva, and Berlin Chemie. PW reports receiving travel grants and speaker fees from Novartis, Ipsen, Recordati, Novo Nordisk, Xeris Pharmaceuticals (Strongbridge), and Lilly. PK reports participation in advisory board meetings by Novo Nordisk, Novartis, and Eli Lilly. AP is an employee of Recordati. AMP was an employee of Recordati when the analyses were conducted. SS has provided consultancy for Novartis and Chiasma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures

Similar articles
-
Pasireotide-induced hyperglycemia in Cushing's disease and Acromegaly: A clinical perspective and algorithms proposal.Front Endocrinol (Lausanne). 2024 Dec 13;15:1455465. doi: 10.3389/fendo.2024.1455465. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39735646 Free PMC article. Review.
-
Managing pasireotide-associated hyperglycemia: a randomized, open-label, Phase IV study.Pituitary. 2021 Dec;24(6):887-903. doi: 10.1007/s11102-021-01161-4. Epub 2021 Jul 18. Pituitary. 2021. PMID: 34275099 Free PMC article. Clinical Trial.
-
Hyperglycemia induced by pasireotide in patients with Cushing's disease or acromegaly.Pituitary. 2016 Oct;19(5):536-43. doi: 10.1007/s11102-016-0734-1. Pituitary. 2016. PMID: 27405306 Free PMC article. Review.
-
Managing hyperglycemia in patients with Cushing's disease treated with pasireotide: medical expert recommendations.Pituitary. 2014 Apr;17(2):180-6. doi: 10.1007/s11102-013-0483-3. Pituitary. 2014. PMID: 23564338 Free PMC article. Review.
-
Management of hyperglycemia associated with pasireotide (SOM230): healthy volunteer study.Diabetes Res Clin Pract. 2014 Mar;103(3):458-65. doi: 10.1016/j.diabres.2013.12.011. Epub 2013 Dec 25. Diabetes Res Clin Pract. 2014. PMID: 24461109 Clinical Trial.
Cited by
-
Pasireotide-induced hyperglycemia in Cushing's disease and Acromegaly: A clinical perspective and algorithms proposal.Front Endocrinol (Lausanne). 2024 Dec 13;15:1455465. doi: 10.3389/fendo.2024.1455465. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39735646 Free PMC article. Review.
-
Medical Treatment of Cushing's Syndrome.Endocrinol Metab (Seoul). 2025 Feb;40(1):26-38. doi: 10.3803/EnM.2024.501. Epub 2025 Jan 13. Endocrinol Metab (Seoul). 2025. PMID: 39801039 Free PMC article. Review.
-
Impact of pasireotide on lipid and glucose metabolism in patients with acromegaly: a systematic review and meta-analysis.J Endocrinol Invest. 2025 Jul 7. doi: 10.1007/s40618-025-02642-0. Online ahead of print. J Endocrinol Invest. 2025. PMID: 40622518 Review.
-
Diabetes mellitus secondary to endocrine diseases: a position statement of the working group of the club of the Italian society of endocrinology (SIE)-Nutrition hormones and metabolism.J Endocrinol Invest. 2025 Aug;48(8):1739-1758. doi: 10.1007/s40618-025-02589-2. Epub 2025 Apr 28. J Endocrinol Invest. 2025. PMID: 40293649 Review.
-
Real-World Experience with Pasireotide-LAR in Cushing's Disease: Single-Center 12-Month Observational Study.J Clin Med. 2025 Apr 18;14(8):2794. doi: 10.3390/jcm14082794. J Clin Med. 2025. PMID: 40283624 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

