Cardiac fibrogenesis: an immuno-metabolic perspective
- PMID: 38577624
- PMCID: PMC10993884
- DOI: 10.3389/fphys.2024.1336551
Cardiac fibrogenesis: an immuno-metabolic perspective
Abstract
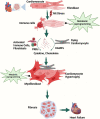
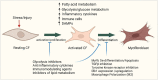
Cardiac fibrosis is a major and complex pathophysiological process that ultimately culminates in cardiac dysfunction and heart failure. This phenomenon includes not only the replacement of the damaged tissue by a fibrotic scar produced by activated fibroblasts/myofibroblasts but also a spatiotemporal alteration of the structural, biochemical, and biomechanical parameters in the ventricular wall, eliciting a reactive remodeling process. Though mechanical stress, post-infarct homeostatic imbalances, and neurohormonal activation are classically attributed to cardiac fibrosis, emerging evidence that supports the roles of immune system modulation, inflammation, and metabolic dysregulation in the initiation and progression of cardiac fibrogenesis has been reported. Adaptive changes, immune cell phenoconversions, and metabolic shifts in the cardiac nonmyocyte population provide initial protection, but persistent altered metabolic demand eventually contributes to adverse remodeling of the heart. Altered energy metabolism, mitochondrial dysfunction, various immune cells, immune mediators, and cross-talks between the immune cells and cardiomyocytes play crucial roles in orchestrating the transdifferentiation of fibroblasts and ensuing fibrotic remodeling of the heart. Manipulation of the metabolic plasticity, fibroblast-myofibroblast transition, and modulation of the immune response may hold promise for favorably modulating the fibrotic response following different cardiovascular pathological processes. Although the immunologic and metabolic perspectives of fibrosis in the heart are being reported in the literature, they lack a comprehensive sketch bridging these two arenas and illustrating the synchrony between them. This review aims to provide a comprehensive overview of the intricate relationship between different cardiac immune cells and metabolic pathways as well as summarizes the current understanding of the involvement of immune-metabolic pathways in cardiac fibrosis and attempts to identify some of the previously unaddressed questions that require further investigation. Moreover, the potential therapeutic strategies and emerging pharmacological interventions, including immune and metabolic modulators, that show promise in preventing or attenuating cardiac fibrosis and restoring cardiac function will be discussed.
Keywords: cardiac fibrosis; cardiometabolism; immune cells; immunometabolism; inflammation; metabolic reprogramming.
Copyright © 2024 Hoque, Gbadegoye, Hassan, Raafat and Lebeche.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abhishek K. J., Huang S. C. C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E., et al. (2015). Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42 (3), 419–430. 10.1016/j.immuni.2015.02.005 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

