Contributions of narrow- and broad-spiking prefrontal and parietal neurons on working memory tasks
- PMID: 38577690
- PMCID: PMC10991738
- DOI: 10.3389/fnsys.2024.1365622
Contributions of narrow- and broad-spiking prefrontal and parietal neurons on working memory tasks
Abstract
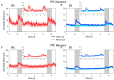
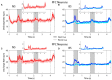
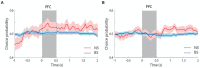
Neurons that generate persistent activity in the primate dorsolateral prefrontal and posterior parietal cortex have been shown to be predictive of behavior in working memory tasks, though subtle differences between them have been observed in how information is represented. The role of different neuron types in each of these areas has not been investigated at depth. We thus compared the activity of neurons classified as narrow-spiking, putative interneurons, and broad-spiking, putative pyramidal neurons, recorded from the dorsolateral prefrontal and posterior parietal cortex of male monkeys, to analyze their role in the maintenance of working memory. Our results demonstrate that narrow-spiking neurons are active during a range of tasks and generate persistent activity during the delay period over which stimuli need to be maintained in memory. Furthermore, the activity of narrow-spiking neurons was predictive of the subject's recall no less than that of broad-spiking neurons, which are exclusively projection neurons in the cortex. Our results show that putative interneurons play an active role during the maintenance of working memory and shed light onto the fundamental neural circuits that determine subjects' memories and judgments.
Keywords: monkey; neurophysiology; posterior parietal cortex; prefrontal cortex; working memory.
Copyright © 2024 Mozumder, Chung, Li and Constantinidis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Functional role of cell classes in monkey prefrontal cortex after learning a working memory task.Commun Biol. 2025 May 6;8(1):703. doi: 10.1038/s42003-025-08142-4. Commun Biol. 2025. PMID: 40328904 Free PMC article.
-
Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex.Front Syst Neurosci. 2010 May 14;4:12. doi: 10.3389/fnsys.2010.00012. eCollection 2010. Front Syst Neurosci. 2010. PMID: 20514341 Free PMC article.
-
Role of Prefrontal Persistent Activity in Working Memory.Front Syst Neurosci. 2016 Jan 5;9:181. doi: 10.3389/fnsys.2015.00181. eCollection 2015. Front Syst Neurosci. 2016. PMID: 26778980 Free PMC article. Review.
-
Single-neuron and population measures of neuronal activity in working memory tasks.J Neurophysiol. 2023 Sep 1;130(3):694-705. doi: 10.1152/jn.00245.2023. Epub 2023 Aug 23. J Neurophysiol. 2023. PMID: 37609703 Free PMC article.
-
Prefrontal cortex and working memory processes.Neuroscience. 2006 Apr 28;139(1):251-61. doi: 10.1016/j.neuroscience.2005.07.003. Epub 2005 Dec 1. Neuroscience. 2006. PMID: 16325345 Review.
Cited by
-
Functional role of cell classes in monkey prefrontal cortex after learning a working memory task.Commun Biol. 2025 May 6;8(1):703. doi: 10.1038/s42003-025-08142-4. Commun Biol. 2025. PMID: 40328904 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

