Heterogeneity of primary and metastatic CAFs: From differential treatment outcomes to treatment opportunities (Review)
- PMID: 38577950
- PMCID: PMC11015919
- DOI: 10.3892/ijo.2024.5642
Heterogeneity of primary and metastatic CAFs: From differential treatment outcomes to treatment opportunities (Review)
Abstract
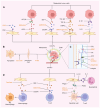
Compared with primary tumor sites, metastatic sites appear more resistant to treatments and respond differently to the treatment regimen. It may be due to the heterogeneity in the microenvironment between metastatic sites and primary tumors. Cancer‑associated fibroblasts (CAFs) are widely present in the tumor stroma as key components of the tumor microenvironment. Primary tumor CAFs (pCAFs) and metastatic CAFs (mCAFs) are heterogeneous in terms of source, activation mode, markers and functional phenotypes. They can shape the tumor microenvironment according to organ, showing heterogeneity between primary tumors and metastases, which may affect the sensitivity of these sites to treatment. It was hypothesized that understanding the heterogeneity between pCAFs and mCAFs can provide a glimpse into the difference in treatment outcomes, providing new ideas for improving the rate of metastasis control in various cancers.
Keywords: heterogeneity; metastatic cancer‑associated fibroblast; primary cancer-associated fibroblast; treatment opportunities; treatment outcomes; tumor microenvironment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
