A CuI6L4 Cage Dynamically Reconfigures to Form Suit[4]anes and Selectively Bind Fluorinated Steroids
- PMID: 38578086
- PMCID: PMC11027141
- DOI: 10.1021/jacs.4c00257
A CuI6L4 Cage Dynamically Reconfigures to Form Suit[4]anes and Selectively Bind Fluorinated Steroids
Abstract
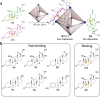
Simple organic ligands can self-assemble with metal ions to generate metal-organic cages, whose cavities bind guests selectively. This binding may enable new methods of chemical separation or sensing, among other useful functions. Here we report the preparation of a CuI6L4 pseudo-octahedral metal-organic cage, the ligands of which self-assemble from simple organic building blocks. Temperature, solvent, and the presence of different guests governed which structure predominated from a dynamic mixture of cage diastereomers with different arrangements of right- or left-handed metal vertices. Dissolution in dimethyl sulfoxide or the binding of tetrahedral guests led to a chiral tetrahedral T-symmetric framework, whereas low temperatures favored the achiral S4-symmetric diastereomer. Tetrahedral guests with long arms were encapsulated to form mechanically bonded suit[4]anes, with guest arms protruding out through host windows. The cage was also observed to bind fluorinated steroids, an important class of drug molecules, but not non-fluorinated steroids, providing the basis for new separation processes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Higher-Order CuI-Based Cages via Subcomponent Self-Assembly.Acc Chem Res. 2025 Apr 15;58(8):1296-1307. doi: 10.1021/acs.accounts.5c00081. Epub 2025 Mar 25. Acc Chem Res. 2025. PMID: 40132056 Free PMC article.
-
Functional Capsules via Subcomponent Self-Assembly.Acc Chem Res. 2018 Oct 16;51(10):2423-2436. doi: 10.1021/acs.accounts.8b00303. Epub 2018 Sep 12. Acc Chem Res. 2018. PMID: 30207688
-
Anionic Templates Drive Conversion between a ZnII9L6 Tricapped Trigonal Prism and ZnII6L4 Pseudo-Octahedra.J Am Chem Soc. 2023 Jul 26;145(29):15990-15996. doi: 10.1021/jacs.3c03981. Epub 2023 Jul 13. J Am Chem Soc. 2023. PMID: 37440669 Free PMC article.
-
Interpenetrated Cage Structures.Chemistry. 2016 Sep 26;22(40):14104-25. doi: 10.1002/chem.201601752. Epub 2016 Jul 15. Chemistry. 2016. PMID: 27417259 Review.
-
Polyanionic Imido-P(V) Ligands: From Transition Metal Complexes to Coordination Driven Self-Assemblies.Chem Rec. 2022 Mar;22(3):e202100281. doi: 10.1002/tcr.202100281. Epub 2021 Dec 27. Chem Rec. 2022. PMID: 34962082 Review.
Cited by
-
Transformation of a Pd6 trifacial barrel to a Pd8 tetrafacial barrel by C70 as guest and oxidative photolysis of alkenes using the C70 encapsulated barrel under red light.Chem Sci. 2025 Jun 5;16(28):12885-12895. doi: 10.1039/d5sc01015b. eCollection 2025 Jul 16. Chem Sci. 2025. PMID: 40521109 Free PMC article.
-
Double-Bridging Increases the Stability of Zinc(II) Metal-Organic Cages.J Am Chem Soc. 2024 Nov 13;146(45):30958-30965. doi: 10.1021/jacs.4c09742. Epub 2024 Nov 4. J Am Chem Soc. 2024. PMID: 39496078 Free PMC article.
-
Dynamic selection in metallo-organic cube CdII 8L4 conformations induced by perfluorooctanoate encapsulation.Chem Sci. 2024 Nov 22;16(1):364-370. doi: 10.1039/d4sc07105k. eCollection 2024 Dec 18. Chem Sci. 2024. PMID: 39620083 Free PMC article.
-
Next-Generation Metal-Organic Frameworks: Shaping the Future of Steroid Compound Management.ACS Omega. 2025 Mar 3;10(10):9890-9902. doi: 10.1021/acsomega.4c11671. eCollection 2025 Mar 18. ACS Omega. 2025. PMID: 40124067 Free PMC article. Review.
-
Higher-Order CuI-Based Cages via Subcomponent Self-Assembly.Acc Chem Res. 2025 Apr 15;58(8):1296-1307. doi: 10.1021/acs.accounts.5c00081. Epub 2025 Mar 25. Acc Chem Res. 2025. PMID: 40132056 Free PMC article.
References
-
- Zhu W.; Guo J.; Ju Y.; Serda R. E.; Croissant J. G.; Shang J.; Coker E.; Agola J. O.; Zhong Q.; Ping Y.; Caruso F.; Brinker C. J. Modular Metal–Organic Polyhedra Superassembly: From Molecular-Level Design to Targeted Drug Delivery. Adv. Mater. 2019, 31 (12), 180677410.1002/adma.201806774. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

