Stabilizing Tumor-Resident Mast Cells Restores T-Cell Infiltration and Sensitizes Sarcomas to PD-L1 Inhibition
- PMID: 38578281
- PMCID: PMC11145177
- DOI: 10.1158/1078-0432.CCR-24-0246
Stabilizing Tumor-Resident Mast Cells Restores T-Cell Infiltration and Sensitizes Sarcomas to PD-L1 Inhibition
Abstract
Purpose: To explore the cellular cross-talk of tumor-resident mast cells (MC) in controlling the activity of cancer-associated fibroblasts (CAF) to overcome tumor microenvironment (TME) abnormalities, enhancing the efficacy of immune-checkpoint inhibitors in sarcoma.
Experimental design: We used a coculture system followed by further validation in mouse models of fibrosarcoma and osteosarcoma with or without administration of the MC stabilizer and antihistamine ketotifen. To evaluate the contribution of ketotifen in sensitizing tumors to therapy, we performed combination studies with doxorubicin chemotherapy and anti-PD-L1 (B7-H1, clone 10F.9G2) treatment. We investigated the ability of ketotifen to modulate the TME in human sarcomas in the context of a repurposed phase II clinical trial.
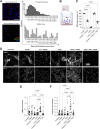
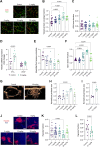
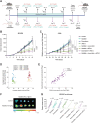
Results: Inhibition of MC activation with ketotifen successfully suppressed CAF proliferation and stiffness of the extracellular matrix accompanied by an increase in vessel perfusion in fibrosarcoma and osteosarcoma as indicated by ultrasound shear wave elastography imaging. The improved tissue oxygenation increased the efficacy of chemoimmunotherapy, supported by enhanced T-cell infiltration and acquisition of tumor antigen-specific memory. Importantly, the effect of ketotifen in reducing tumor stiffness was further validated in sarcoma patients, highlighting its translational potential.
Conclusions: Our study suggests the targeting of MCs with clinically administered drugs, such as antihistamines, as a promising approach to overcome resistance to immunotherapy in sarcomas.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Stubbs M, McSheehy PM, Griffiths JR, Bashford CL. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today 2000;6:15–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

