Administration of Gas6 attenuates lung fibrosis via inhibition of the epithelial-mesenchymal transition and fibroblast activation
- PMID: 38578518
- PMCID: PMC10997547
- DOI: 10.1007/s10565-024-09858-5
Administration of Gas6 attenuates lung fibrosis via inhibition of the epithelial-mesenchymal transition and fibroblast activation
Abstract
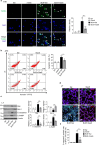
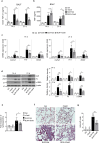
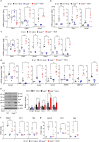
The epithelial-mesenchymal transition (EMT) and fibroblast activation are major events in idiopathic pulmonary fibrosis pathogenesis. Here, we investigated whether growth arrest-specific protein 6 (Gas6) plays a protective role in lung fibrosis via suppression of the EMT and fibroblast activation. rGas6 administration inhibited the EMT in isolated mouse ATII cells 14 days post-BLM treatment based on morphologic cellular alterations, changes in mRNA and protein expression profiles of EMT markers, and induction of EMT-activating transcription factors. BLM-induced increases in gene expression of fibroblast activation-related markers and the invasive capacity of primary lung fibroblasts in primary lung fibroblasts were reversed by rGas6 administration. Furthermore, the hydroxyproline content and collagen accumulation in interstitial areas with damaged alveolar structures in lung tissue were reduced by rGas6 administration. Targeting Gas6/Axl signaling events with specific inhibitors of Axl (BGB324), COX-2 (NS-398), EP1/EP2 receptor (AH-6809), or PGD2 DP2 receptor (BAY-u3405) reversed the inhibitory effects of rGas6 on EMT and fibroblast activation. Finally, we confirmed the antifibrotic effects of Gas6 using Gas6-/- mice. Therefore, Gas6/Axl signaling events play a potential role in inhibition of EMT process and fibroblast activation via COX-2-derived PGE2 and PGD2 production, ultimately preventing the development of pulmonary fibrosis.
Keywords: Axl; EMT; Fibroblast activation; Gas6; Pulmonary fibrosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Gas6 Prevents Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells via Production of PGE2, PGD2 and Their Receptors.Cells. 2019 Jun 26;8(7):643. doi: 10.3390/cells8070643. Cells. 2019. PMID: 31247991 Free PMC article.
-
Targeting of TAM Receptors Ameliorates Fibrotic Mechanisms in Idiopathic Pulmonary Fibrosis.Am J Respir Crit Care Med. 2018 Jun 1;197(11):1443-1456. doi: 10.1164/rccm.201707-1519OC. Am J Respir Crit Care Med. 2018. PMID: 29634284 Free PMC article.
-
RhoA-Dependent HGF and c-Met Mediate Gas6-Induced Inhibition of Epithelial-Mesenchymal Transition, Migration, and Invasion of Lung Alveolar Epithelial Cells.Biomolecules. 2019 Oct 4;9(10):565. doi: 10.3390/biom9100565. Biomolecules. 2019. PMID: 31590238 Free PMC article.
-
Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation.J Hepatol. 2015 Sep;63(3):670-8. doi: 10.1016/j.jhep.2015.04.013. Epub 2015 Apr 20. J Hepatol. 2015. PMID: 25908269 Free PMC article.
-
Non-coding RNAs and nuclear architecture during epithelial-mesenchymal transition in lung cancer and idiopathic pulmonary fibrosis.Cell Signal. 2020 Jun;70:109593. doi: 10.1016/j.cellsig.2020.109593. Epub 2020 Mar 2. Cell Signal. 2020. PMID: 32135188 Review.
Cited by
-
Recent advances in TAM mechanisms in lung diseases.J Transl Med. 2025 Apr 26;23(1):479. doi: 10.1186/s12967-025-06398-2. J Transl Med. 2025. PMID: 40287707 Free PMC article. Review.
-
Assessment of tumor biomarkers for prognosis in interstitial lung disease associated with connective tissue disease: a prospective study.J Thorac Dis. 2024 Nov 30;16(11):7383-7396. doi: 10.21037/jtd-24-922. Epub 2024 Nov 21. J Thorac Dis. 2024. PMID: 39678894 Free PMC article.
-
TGF-β/SMAD Pathway Mediates Cadmium Poisoning-Induced Chicken Liver Fibrosis and Epithelial-Mesenchymal Transition.Biol Trace Elem Res. 2025 Apr;203(4):2295-2309. doi: 10.1007/s12011-024-04294-2. Epub 2024 Jul 3. Biol Trace Elem Res. 2025. PMID: 38958867
-
Gas6 induces AIM to suppress acute lung injury in mice by inhibiting NLRP3 inflammasome activation and inducing autophagy.Front Immunol. 2025 Feb 17;16:1523166. doi: 10.3389/fimmu.2025.1523166. eCollection 2025. Front Immunol. 2025. PMID: 40034700 Free PMC article.
References
-
- Bärnthaler T, Theiler A, Zabini D, Trautmann S, Stacher-Priehse E, Lanz I, et al. Inhibiting eicosanoid degradation exerts antifibrotic effects in a pulmonary fibrosis mouse model and human tissue. J Allergy Clin Immunol. 2020;145(3):818-33.e11. - PubMed
-
- Bortnick AE, Favari E, Tao JQ, Francone OL, Reilly M, Zhang Y, et al. Identification and characterization of rodent ABCA1 in isolated type II pneumocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285(4):L869–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

