Drug Shortages Prior to and During the COVID-19 Pandemic
- PMID: 38578641
- PMCID: PMC10998160
- DOI: 10.1001/jamanetworkopen.2024.4246
Drug Shortages Prior to and During the COVID-19 Pandemic
Abstract
Importance: Drug shortages are a chronic and worsening issue that compromises patient safety. Despite the destabilizing impact of the COVID-19 pandemic on pharmaceutical production, it remains unclear whether issues affecting the drug supply chain were more likely to result in meaningful shortages during the pandemic.
Objective: To estimate the proportion of supply chain issue reports associated with drug shortages overall and with the COVID-19 pandemic.
Design, setting, and participants: This longitudinal cross-sectional study used data from the IQVIA Multinational Integrated Data Analysis database, comprising more than 85% of drug purchases by US pharmacies from wholesalers and manufacturers, from 2017 to 2021. Data were analyzed from January to May 2023.
Exposure: Presence of a supply chain issue report to the US Food and Drug Administration or the American Society of Health-Systems Pharmacists (ASHP).
Main outcomes and measures: The main outcome was drug shortage, defined as at least 33% decrease in units purchased within 6 months of a supply chain issue report. Random-effects logistic regression models compared the marginal odds of shortages for drugs with vs without reports. Interaction terms assessed heterogeneity prior to vs during the COVID-19 pandemic and by drug characteristics (formulation, age, essential medicine status, clinician- vs self-administered, sales volume, and number of manufacturers).
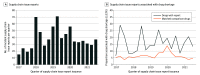
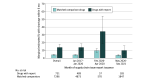
Results: A total of 571 drugs exposed to 731 supply chain issue reports were matched to 7296 comparison medications with no reports. After adjusting for drug characteristics, 13.7% (95% CI, 10.4%-17.8%) of supply chain issue reports were associated with subsequent drug shortages vs 4.1% (95% CI, 3.6%-4.8%) of comparators (marginal odds ratio [mOR], 3.7 [95% CI, 2.6-5.1]). Shortages increased among both drugs with and without reports in February to April 2020 (34.2% of drugs with supply chain issue reports and 9.5% of comparison drugs; mOR, 4.9 [95% CI, 2.1-11.6]), and then decreased after May 2020 (9.8% of drugs with reports and 3.6% of comparison drugs; mOR, 2.9 [95% CI, 1.6-5.3]). Significant associations were identified by formulation (parenteral mOR, 1.9 [95% CI, 1.1-3.2] vs oral mOR, 5.4 [95% CI, 3.3-8.8]; P for interaction = .008), WHO essential medicine status (essential mOR, 2.2 [95% CI, 1.3-5.2] vs nonessential mOR, 4.6 [95% CI, 3.2-6.7]; P = .02), and for brand-name vs generic status (brand-name mOR, 8.1 [95% CI, 4.0-16.0] vs generic mOR, 2.4 [95% CI, 1.7-3.6]; P = .002).
Conclusions and relevance: In this national cross-sectional study, supply chain issues associated with drug shortages increased at the beginning of the COVID-19 pandemic. Ongoing policy work is needed to protect US drug supplies from future shocks and to prioritize clinically valuable drugs at greatest shortage risk.
Conflict of interest statement
Figures


Comment in
-
Drug Shortages-A Study in Complexity.JAMA Netw Open. 2024 Apr 1;7(4):e244168. doi: 10.1001/jamanetworkopen.2024.4168. JAMA Netw Open. 2024. PMID: 38578644 No abstract available.
References
-
- American Society of Health System Pharmacists . Drug Shortages Statistics. Published 2023. Accessed May 24, 2023. https://www.ashp.org/Drug-Shortages/Shortage-Resources/Drug-Shortages-St...
-
- Food and Drug Administration Safety and Innovation Act (FDASIA). Pub Law No. 114-144. 126 Stat 993 (2012). Accessed May 23, 2023. https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

