Impact of improved dead time correction on the quantification accuracy of a dedicated BrainPET scanner
- PMID: 38578749
- PMCID: PMC10997125
- DOI: 10.1371/journal.pone.0296357
Impact of improved dead time correction on the quantification accuracy of a dedicated BrainPET scanner
Abstract
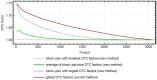
Objective: Quantitative values derived from PET brain images are of high interest for neuroscientific applications. Insufficient DT correction (DTC) can lead to a systematic bias of the output parameters obtained by a detailed analysis of the time activity curves (TACs). The DTC method currently used for the Siemens 3T MR BrainPET insert is global, i.e., differences in DT losses between detector blocks are not considered, leading to inaccurate DTC and, consequently, to inaccurate measurements masked by a bias. However, following careful evaluation with phantom measurements, a new block-pairwise DTC method has demonstrated a higher degree of accuracy compared to the global DTC method.
Approach: Differences between the global and the block-pairwise DTC method were studied in this work by applying several radioactive tracers. We evaluated the impact on [11C]ABP688, O-(2-[18F]fluoroethyl)-L-tyrosine (FET), and [15O]H2O TACs.
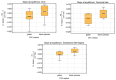
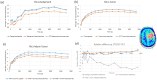
Results: For [11C]ABP688, a relevant bias of between -0.0034 and -0.0053 ml/ (cm3 • min) was found in all studied brain regions for the volume of distribution (VT) when using the current global DTC method. For [18F]FET-PET, differences of up to 10% were observed in the tumor-to-brain ratio (TBRmax), these differences depend on the radial distance of the maximum from the PET isocenter. For [15O]H2O, differences between +4% and -7% were observed in the GM region. Average biases of -4.58%, -3.2%, and -1.2% for the regional cerebral blood flow (CBF (K1)), the rate constant k2, and the volume of distribution VT were observed, respectively. Conversely, in the white matter region, average biases of -4.9%, -7.0%, and 3.8% were observed for CBF (K1), k2, and VT, respectively.
Conclusion: The bias introduced by the global DTC method leads to an overestimation in the studied quantitative parameters for all applications compared to the block-pairwise method.
Significance: The observed differences between the two DTC methods are particularly relevant for research applications in neuroscientific studies as they affect the accuracy of quantitative Brain PET images.
Copyright: © 2024 Issa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
A detector block-pairwise dead time correction method for improved quantitation with a dedicated BrainPET scanner.Phys Med Biol. 2022 Dec 2;67(23). doi: 10.1088/1361-6560/aca1f3. Phys Med Biol. 2022. PMID: 36356317
-
Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging.J Cereb Blood Flow Metab. 2014 Aug;34(8):1373-80. doi: 10.1038/jcbfm.2014.92. Epub 2014 May 21. J Cereb Blood Flow Metab. 2014. PMID: 24849665 Free PMC article.
-
Comparison of fluorine-18 and bromine-76 imaging in positron emission tomography.Eur J Nucl Med. 1999 Jul;26(7):758-66. doi: 10.1007/s002590050447. Eur J Nucl Med. 1999. PMID: 10398824
-
On the quantification accuracy, homogeneity, and stability of simultaneous positron emission tomography/magnetic resonance imaging systems.Invest Radiol. 2014 Jun;49(6):373-81. doi: 10.1097/RLI.0000000000000021. Invest Radiol. 2014. PMID: 24368614
-
Brain PET and functional MRI: why simultaneously using hybrid PET/MR systems?Q J Nucl Med Mol Imaging. 2017 Dec;61(4):345-359. doi: 10.23736/S1824-4785.17.03008-4. Epub 2017 Jul 27. Q J Nucl Med Mol Imaging. 2017. PMID: 28750494 Review.
References
-
- Freedman NM, Bacharach SL, McCord ME, Bonow RO. "Spatially dependent deadtime losses in high count rate cardiac PET." Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine 33.12 (1992): 2226–2231. . - PubMed
-
- Bailey D.L., Maisey M.N., Townsend D.W. and Valk P.E., 2005. Positron emission tomography. Vol. 2. London: Springer, 2005.
-
- Knoll Glenn F. Radiation detection and measurement. John Wiley & Sons, 2010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

