β-Cell Insulin Resistance Plays a Causal Role in Fat-Induced β-Cell Dysfunction In Vitro and In Vivo
- PMID: 38578954
- PMCID: PMC11033845
- DOI: 10.1210/endocr/bqae044
β-Cell Insulin Resistance Plays a Causal Role in Fat-Induced β-Cell Dysfunction In Vitro and In Vivo
Abstract
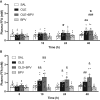
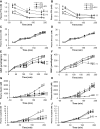
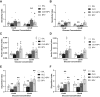
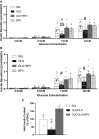
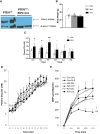
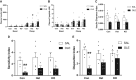
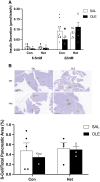
In the classical insulin target tissues of liver, muscle, and adipose tissue, chronically elevated levels of free fatty acids (FFA) impair insulin signaling. Insulin signaling molecules are also present in β-cells where they play a role in β-cell function. Therefore, inhibition of the insulin/insulin-like growth factor 1 pathway may be involved in fat-induced β-cell dysfunction. To address the role of β-cell insulin resistance in FFA-induced β-cell dysfunction we co-infused bisperoxovanadate (BPV) with oleate or olive oil for 48 hours in rats. BPV, a tyrosine phosphatase inhibitor, acts as an insulin mimetic and is devoid of any antioxidant effect that could prevent β-cell dysfunction, unlike most insulin sensitizers. Following fat infusion, rats either underwent hyperglycemic clamps for assessment of β-cell function in vivo or islets were isolated for ex vivo assessment of glucose-stimulated insulin secretion (GSIS). We also incubated islets with oleate or palmitate and BPV for in vitro assessment of GSIS and Akt (protein kinase B) phosphorylation. Next, mice with β-cell specific deletion of PTEN (phosphatase and tensin homolog; negative regulator of insulin signaling) and littermate controls were infused with oleate for 48 hours, followed by hyperglycemic clamps or ex vivo evaluation of GSIS. In rat experiments, BPV protected against fat-induced impairment of β-cell function in vivo, ex vivo, and in vitro. In mice, β-cell specific deletion of PTEN protected against oleate-induced β-cell dysfunction in vivo and ex vivo. These data support the hypothesis that β-cell insulin resistance plays a causal role in FFA-induced β-cell dysfunction.
Keywords: beta-cell dysfunction; beta-cell insulin resistance; beta-cell insulin signaling; lipotoxicity; oleate; olive oil.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Xiao C, Giacca A, Lewis GF. Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia 2008;51(1):139‐146. - PubMed
-
- Böni-Schnetzler M, Boller S, Debray S, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 2009;150(12):5218‐5229. - PubMed
-
- Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 2002;51(7):2005‐2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

