Biofunctional lipid nanoparticles for precision treatment and prophylaxis of bacterial infections
- PMID: 38578994
- PMCID: PMC10997193
- DOI: 10.1126/sciadv.adk9754
Biofunctional lipid nanoparticles for precision treatment and prophylaxis of bacterial infections
Abstract
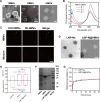
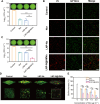
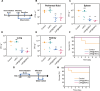
The lack of bacterial-targeting function in antibiotics and their prophylactic usage have caused overuse of antibiotics, which lead to antibiotic resistance and inevitable long-term toxicity. To overcome these issues, we develop neutrophil-bacterial hybrid cell membrane vesicle (HMV)-coated biofunctional lipid nanoparticles (LNP@HMVs), which are designed to transport antibiotics specifically to bacterial cells at the infection site for the effective treatment and prophylaxis of bacterial infection. The dual targeting ability of HMVs to inflammatory vascular endothelial cells and homologous Gram-negative bacterial cells results in targeted accumulation of LNP@HMVs in the site of infections. LNP@HMVs loaded with the antibiotic norfloxacin not only exhibit enhanced activity against planktonic bacteria and bacterial biofilms in vitro but also achieve potent therapeutic efficacy in treating both systemic infection and lung infection. Furthermore, LNP@HMVs trigger the activation of specific humoral and cellular immunity to prevent bacterial infection. Together, LNP@HMVs provide a promising strategy to effectively treat and prevent bacterial infection.
Figures




References
-
- Feng W. L., Li G. F., Kang X. X., Wang R. B., Liu F., Zhao D. D., Li H. F., Bu F. Q., Yu Y. J., Moriarty T. F., Ren Q., Wang X., Cascade-targeting poly(amino acid) nanoparticles eliminate intracellular bacteria via on-site antibiotic delivery. Adv. Mater. 34, e2109789 (2022). - PubMed
-
- Wu Y. M., Lin Y. F., Cong Z. H., Chen K., Xiao X. M., Wu X., Liu L. Q., She Y. R., Liu S. Q., Zhou R. Y., Yin G., Shao X. Y., Dai Y. D., Lin H. D., Liu R. H., Peptide polymer-doped cement acting as an effective treatment of MRSA-infected chronic osteomyelitis. Adv. Funct. Mater. 32, 2107942 (2022).
-
- Huang D., Li D. W., Wang T. T., Shen H., Zhao P., Liu B. X., You Y. Z., Ma Y. Z., Yang F., Wu D. C., Wang S. G., Isoniazid conjugated poly(lactide-co-glycolide): Long-term controlled drug release and tissue regeneration for bone tuberculosis therapy. Biomaterials 52, 417–425 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

