IL-18-secreting multiantigen targeting CAR T cells eliminate antigen-low myeloma in an immunocompetent mouse model
- PMID: 38579288
- PMCID: PMC11302468
- DOI: 10.1182/blood.2023022293
IL-18-secreting multiantigen targeting CAR T cells eliminate antigen-low myeloma in an immunocompetent mouse model
Abstract
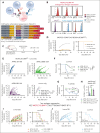
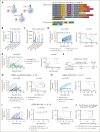
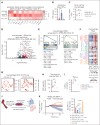
Multiple myeloma is a plasma cell malignancy that is currently incurable with conventional therapies. Following the success of CD19-targeted chimeric antigen receptor (CAR) T cells in leukemia and lymphoma, CAR T cells targeting B-cell maturation antigen (BCMA) more recently demonstrated impressive activity in relapsed and refractory myeloma patients. However, BCMA-directed therapy can fail due to weak expression of BCMA on myeloma cells, suggesting that novel approaches to better address this antigen-low disease may improve patient outcomes. We hypothesized that engineered secretion of the proinflammatory cytokine interleukin-18 (IL-18) and multiantigen targeting could improve CAR T-cell activity against BCMA-low myeloma. In a syngeneic murine model of myeloma, CAR T cells targeting the myeloma-associated antigens BCMA and B-cell activating factor receptor (BAFF-R) failed to eliminate myeloma when these antigens were weakly expressed, whereas IL-18-secreting CAR T cells targeting these antigens promoted myeloma clearance. IL-18-secreting CAR T cells developed an effector-like T-cell phenotype, promoted interferon-gamma production, reprogrammed the myeloma bone marrow microenvironment through type-I/II interferon signaling, and activated macrophages to mediate antimyeloma activity. Simultaneous targeting of weakly-expressed BCMA and BAFF-R with dual-CAR T cells enhanced T-cell:target-cell avidity, increased overall CAR signal strength, and stimulated antimyeloma activity. Dual-antigen targeting augmented CAR T-cell secretion of engineered IL-18 and facilitated elimination of larger myeloma burdens in vivo. Our results demonstrate that combination of engineered IL-18 secretion and multiantigen targeting can eliminate myeloma with weak antigen expression through distinct mechanisms.
Conflict of interest statement
Conflict-of-interest disclosure: A.P.B. has received compensation for participating in consulting activities with Bristol Myers Squibb. S.E.J. and M.R.M.v.d.B. are coinventors on patent applications related to this work. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics and stock options from Notch Therapeutics and Pluto Therapeutics; he has received royalties from Wolters Kluwer; has consulted, received honorarium from or participated in advisory boards for Seres Therapeutics, Vor Biopharma, Rheos Medicines, Frazier Healthcare Partners, Nektar Therapeutics, Notch Therapeutics, Ceramedix, Lygenesis, Pluto Therapeutics, GlaskoSmithKline, Da Volterra, Thymofox, Garuda, Novartis (spouse), Synthekine (spouse), BeiGene (spouse), Kite (spouse); has intellectual property licensing with Seres Therapeutics and Juno Therapeutics; and holds a fiduciary role on the foundation board of Deutsche Knochenmarkspenderdatei (a nonprofit organization). Memorial Sloan Kettering Cancer Center has institutional financial interests relative to Seres Therapeutics. B.B. is leader of the scientific advisory board in the Nykode Therapeutics (former Vaccibody AS) company in which he holds shares. The remaining authors declare no competing financial interests.
Figures




References
-
- Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–348. - PubMed
-
- Cancer Stat Facts: Myeloma. National Cancer Institute. https://seer.cancer.gov/statfacts/html/mulmy.html
-
- Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076–3084. - PubMed
-
- Mikkilineni L, Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol. 2021;18(2):71–84. - PubMed
-
- Van De Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131(1):13–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

