Clustered de novo start-loss variants in GLUL result in a developmental and epileptic encephalopathy via stabilization of glutamine synthetase
- PMID: 38579670
- PMCID: PMC11023914
- DOI: 10.1016/j.ajhg.2024.03.005
Clustered de novo start-loss variants in GLUL result in a developmental and epileptic encephalopathy via stabilization of glutamine synthetase
Abstract
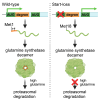
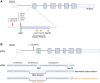
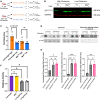
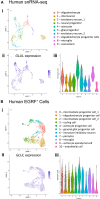
Glutamine synthetase (GS), encoded by GLUL, catalyzes the conversion of glutamate to glutamine. GS is pivotal for the generation of the neurotransmitters glutamate and gamma-aminobutyric acid and is the primary mechanism of ammonia detoxification in the brain. GS levels are regulated post-translationally by an N-terminal degron that enables the ubiquitin-mediated degradation of GS in a glutamine-induced manner. GS deficiency in humans is known to lead to neurological defects and death in infancy, yet how dysregulation of the degron-mediated control of GS levels might affect neurodevelopment is unknown. We ascertained nine individuals with severe developmental delay, seizures, and white matter abnormalities but normal plasma and cerebrospinal fluid biochemistry with de novo variants in GLUL. Seven out of nine were start-loss variants and two out of nine disrupted 5' UTR splicing resulting in splice exclusion of the initiation codon. Using transfection-based expression systems and mass spectrometry, these variants were shown to lead to translation initiation of GS from methionine 18, downstream of the N-terminal degron motif, resulting in a protein that is stable and enzymatically competent but insensitive to negative feedback by glutamine. Analysis of human single-cell transcriptomes demonstrated that GLUL is widely expressed in neuro- and glial-progenitor cells and mature astrocytes but not in post-mitotic neurons. One individual with a start-loss GLUL variant demonstrated periventricular nodular heterotopia, a neuronal migration disorder, yet overexpression of stabilized GS in mice using in utero electroporation demonstrated no migratory deficits. These findings underline the importance of tight regulation of glutamine metabolism during neurodevelopment in humans.
Keywords: GLUL; degron motif; epileptic encephalopathies; glutamine metabolism; glutamine synthetase.
Copyright © 2024 American Society of Human Genetics. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Palladino A.A., Stanley C.A. The hyperinsulinism/hyperammonemia syndrome. Rev. Endocr. Metab. Disord. 2010;11:171–178. - PubMed
-
- Tsuchida N., Hamada K., Shiina M., Kato M., Kobayashi Y., Tohyama J., Kimura K., Hoshino K., Ganesan V., Teik K.W., et al. GRIN2D variants in three cases of developmental and epileptic encephalopathy. Clin. Genet. 2018;94:538–547. - PubMed
-
- Li D., Yuan H., Ortiz-Gonzalez X.R., Marsh E.D., Tian L., McCormick E.M., Kosobucki G.J., Chen W., Schulien A.J., Chiavacci R., et al. GRIN2D Recurrent De Novo Dominant Mutation Causes a Severe Epileptic Encephalopathy Treatable with NMDA Receptor Channel Blockers. Am. J. Hum. Genet. 2016;99:802–816. - PMC - PubMed
-
- Rumping L., Tessadori F., Pouwels P.J.W., Vringer E., Wijnen J.P., Bhogal A.A., Savelberg S.M.C., Duran K.J., Bakkers M.J.G., Ramos R.J.J., et al. GLS hyperactivity causes glutamate excess, infantile cataract and profound developmental delay. Hum. Mol. Genet. 2019;28:96–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

