Receptor transfer between immune cells by autoantibody-enhanced, CD32-driven trogocytosis is hijacked by HIV-1 to infect resting CD4 T cells
- PMID: 38579727
- PMCID: PMC11031382
- DOI: 10.1016/j.xcrm.2024.101483
Receptor transfer between immune cells by autoantibody-enhanced, CD32-driven trogocytosis is hijacked by HIV-1 to infect resting CD4 T cells
Abstract
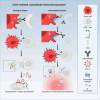
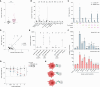
Immune cell phenotyping frequently detects lineage-unrelated receptors. Here, we report that surface receptors can be transferred from primary macrophages to CD4 T cells and identify the Fcγ receptor CD32 as driver and cargo of this trogocytotic transfer. Filamentous CD32+ nanoprotrusions deposit distinct plasma membrane patches onto target T cells. Transferred receptors confer cell migration and adhesion properties, and macrophage-derived membrane patches render resting CD4 T cells susceptible to infection by serving as hotspots for HIV-1 binding. Antibodies that recognize T cell epitopes enhance CD32-mediated trogocytosis. Such autoreactive anti-HIV-1 envelope antibodies can be found in the blood of HIV-1 patients and, consistently, the percentage of CD32+ CD4 T cells is increased in their blood. This CD32-mediated, antigen-independent cell communication mode transiently expands the receptor repertoire and functionality of immune cells. HIV-1 hijacks this mechanism by triggering the generation of trogocytosis-promoting autoantibodies to gain access to immune cells critical to its persistence.
Keywords: CD32; CRISPR-Cas9; HIV reservoir; autoantibodies; immune cell communication; trogocytosis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

