Preclinical development of novel PD-L1 tracers and first-in-human study of [68Ga]Ga-NOTA-RW102 in patients with lung cancers
- PMID: 38580333
- PMCID: PMC11002357
- DOI: 10.1136/jitc-2024-008794
Preclinical development of novel PD-L1 tracers and first-in-human study of [68Ga]Ga-NOTA-RW102 in patients with lung cancers
Abstract
Background: The programmed cell death protein-1 (PD-1)/programmed death receptor ligand 1 (PD-L1) axis critically facilitates cancer cells' immune evasion. Antibody therapeutics targeting the PD-1/PD-L1 axis have shown remarkable efficacy in various tumors. Immuno-positron emission tomography (ImmunoPET) imaging of PD-L1 expression may help reshape solid tumors' immunotherapy landscape.
Methods:
By immunizing an alpaca with recombinant human PD-L1, three clones of the
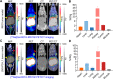
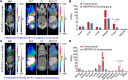
Results: While RW102 has a high binding affinity to PD-L1 with an excellent KD value of 15.29 pM, ABDRW102 simultaneously binds to human PD-L1 and human serum albumin with an excellent KD value of 3.71 pM and 3.38 pM, respectively. Radiotracers derived from RW102 and ABDRW102 have different in vivo circulation times. In preclinical studies, [68Ga]Ga-NOTA-RW102 immunoPET imaging allowed same-day annotation of differential PD-L1 expression with specificity, while [64Cu]Cu-NOTA-ABDRW102 and [89Zr]Zr-DFO-ABDRW102 enabled longitudinal visualization of PD-L1. More importantly, a pilot clinical trial shows the safety and diagnostic value of [68Ga]Ga-NOTA-RW102 immunoPET imaging in patients with NSCLCs and its potential to predict immune-related adverse effects following PD-L1-targeted immunotherapies.
Conclusions: We developed and validated a series of PD-L1-targeted tracers. Initial preclinical and clinical evidence indicates that immunoPET imaging with [68Ga]Ga-NOTA-RW102 holds promise in visualizing differential PD-L1 expression, selecting patients for PD-L1-targeted immunotherapies, and monitoring immune-related adverse effects in patients receiving PD-L1-targeted treatments.
Trial registration number: NCT06165874.
Keywords: Biomarker; Lung Cancer; Nuclear medicine; fMRI / PET.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: WW, YZ and JL are inventors of a pending patent describing the reported PD-L1 tracers. WW is a consultant of Alpha Nuclide (Ningbo) Medical Technology Co., Ltd. WC declares conflict of interest with the following corporations: Actithera, Inc., Portrai, Inc., rTR Technovation Corporation, Four Health Global Pharmaceuticals Inc., and POP Biotechnologies, Inc.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
