Obesity is a risk factor for poor response to treatment in early rheumatoid arthritis: a NORD-STAR study
- PMID: 38580350
- PMCID: PMC11148705
- DOI: 10.1136/rmdopen-2024-004227
Obesity is a risk factor for poor response to treatment in early rheumatoid arthritis: a NORD-STAR study
Abstract
Objective: This report from the NORD-STAR (Nordic Rheumatic Diseases Strategy Trials and Registries) trial aimed to determine if obesity is associated with response to conventional and biological antirheumatic treatment in early rheumatoid arthritis (RA).
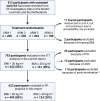
Methods: This report included 793 participants with untreated early RA from the randomised, longitudinal NORD-STAR trial, all of whom had their body mass index (BMI) assessed at baseline. Obesity was defined as BMI ≥30 kg/m2. All participants were randomised 1:1:1:1 to one of four treatment arms: active conventional treatment, certolizumab-pegol, abatacept and tocilizumab. Clinical and laboratory measurements were performed at baseline and at 8, 12, 24 and 48-week follow-up. The primary endpoint for this report was response to treatment based on Clinical Disease Activity Index (CDAI) and Simple Disease Activity Index (SDAI) remission and Disease Activity Score with 28 joints using C-reactive protein (DAS28-CRP) <2.6 stratified by BMI.
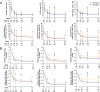
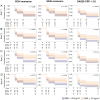
Results: Out of 793 people included in the present report, 161 (20%) had obesity at baseline. During follow-up, participants with baseline obesity had higher disease activity compared with those with lower BMI, despite having similar disease activity at baseline. In survival analyses, obesity was associated with a lower likelihood of achieving response to treatment during follow-up for up to 48 weeks (CDAI remission, HR 0.84, 95% CI 0.67 to 1.05; SDAI, HR 0.77, 95% CI 0.62 to 0.97; DAS28-CRP <2.6, HR 0.78, 95% CI 0.64 to 0.95). The effect of obesity on response to treatment was not influenced by the treatment arms.
Conclusion: In people with untreated early RA followed up for up to 48 weeks, obesity was associated with a lower likelihood of good treatment response, irrespective of the type of randomised treatment received.
Trial registration number: NCT01491815.
Keywords: Rheumatoid Arthritis; Risk Factors; Treatment.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MLH reports research grants from AbbVie, iogen, BMS, Celltrion, Eli Lilly, Janssen Biologics B.V., Lundbeck Foundation, MSD, Pfizer, Roche, Samsung Biopies, Sandoz, Novartis, Nordforsk to institution; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pfizer, Medac, Sandoz paid to institution; participation on a Data Safety Monitoring Board or Advisory Board from AbbVie paid to institution; MLH has chaired the steering committee of the Danish Rheumatology Quality Registry (DANBIO, DRQ), which receives public funding from the hospital owners and funding from pharmaceutical companies; MLH co-chairs EuroSpA, which generates real-world evidence of treatment of psoriatic arthritis and axial spondylarthritis based on secondary data and is partly funded by Novartis. DN reports personal consultancy fees from BMS, Lilly, MSD, Novartis, Pfizer and UCB, and personal study grant from MSD, outside current work. BG reports consulting fee from Novartis and Lectures fees from Novartis and Nordic Pharma. MØ reports grants from Amgen, BMS, Merck, Celgene and Novartis to institution; consulting fees from Galapagos, Gilead, Hospira, Janssen, Merck, Novartis, Pfizer, UCB; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, BMS, Eli-Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, UCB; support for attending meetings and/or travel from UCB; participation on a Data Safety Monitoring Board or Advisory Board from Galapagos, Gilead, Hospira, Janssen, Merck, Novartis, Pfizer, UCB. TS-I reports research grant from Amgen paid to the institution, honoraria from Nordic Pharma. TU reports personal fees from Galapagos, Lilly, Pfizer, UCB outside the submitted work.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
