Fluorescently labelled vedolizumab to visualise drug distribution and mucosal target cells in inflammatory bowel disease
- PMID: 38580386
- PMCID: PMC11347245
- DOI: 10.1136/gutjnl-2023-331696
Fluorescently labelled vedolizumab to visualise drug distribution and mucosal target cells in inflammatory bowel disease
Abstract
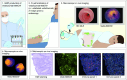
Objective: Improving patient selection and development of biological therapies such as vedolizumab in IBD requires a thorough understanding of the mechanism of action and target binding, thereby providing individualised treatment strategies. We aimed to visualise the macroscopic and microscopic distribution of intravenous injected fluorescently labelled vedolizumab, vedo-800CW, and identify its target cells using fluorescence molecular imaging (FMI).
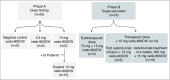
Design: Forty three FMI procedures were performed, which consisted of macroscopic in vivo assessment during endoscopy, followed by macroscopic and microscopic ex vivo imaging. In phase A, patients received an intravenous dose of 4.5 mg, 15 mg vedo-800CW or no tracer prior to endoscopy. In phase B, patients received 15 mg vedo-800CW preceded by an unlabelled (sub)therapeutic dose of vedolizumab.
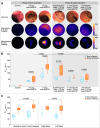
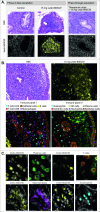
Results: FMI quantification showed a dose-dependent increase in vedo-800CW fluorescence intensity in inflamed tissues, with 15 mg (153.7 au (132.3-163.7)) as the most suitable tracer dose compared with 4.5 mg (55.3 au (33.6-78.2)) (p=0.0002). Moreover, the fluorescence signal decreased by 61% when vedo-800CW was administered after a therapeutic dose of unlabelled vedolizumab, suggesting target saturation in the inflamed tissue. Fluorescence microscopy and immunostaining showed that vedolizumab penetrated the inflamed mucosa and was associated with several immune cell types, most prominently with plasma cells.
Conclusion: These results indicate the potential of FMI to determine the local distribution of drugs in the inflamed target tissue and identify drug target cells, providing new insights into targeted agents for their use in IBD.
Trial registration number: NCT04112212.
Keywords: ANTIBODY TARGETED THERAPY; FLUORESCENCE ENDOSCOPY; IBD; PHARMACOKINETICS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GD received research grants from Royal DSM, Takeda and Janssen Pharmaceuticals and speaker fees from AbbVie, Pfizer, Takeda and Janssen Pharmaceuticals. EAMF is supported by a ZonMW Clinical Fellowship grant (project number 90719075) and has received an unrestricted research grant from Takeda. MD and SJ are employees and shareholders of Regeneron Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
