Senescent-like macrophages mediate angiogenesis for endplate sclerosis via IL-10 secretion in male mice
- PMID: 38580630
- PMCID: PMC10997778
- DOI: 10.1038/s41467-024-47317-1
Senescent-like macrophages mediate angiogenesis for endplate sclerosis via IL-10 secretion in male mice
Abstract
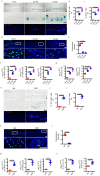
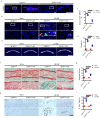
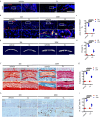
Endplate sclerosis is a notable aspect of spine degeneration or aging, but the mechanisms remain unclear. Here, we report that senescent macrophages accumulate in the sclerotic endplates of lumbar spine instability (LSI) or aging male mouse model. Specifically, knockout of cdkn2a (p16) in macrophages abrogates LSI or aging-induced angiogenesis and sclerosis in the endplates. Furthermore, both in vivo and in vitro studies indicate that IL-10 is the primary elevated cytokine of senescence-related secretory phenotype (SASP). Mechanistically, IL-10 increases pSTAT3 in endothelial cells, leading to pSTAT3 directly binding to the promoters of Vegfa, Mmp2, and Pdgfb to encourage their production, resulting in angiogenesis. This study provides information on understanding the link between immune senescence and endplate sclerosis, which might be useful for therapeutic approaches.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

