Concomitant medication, comorbidity and survival in patients with breast cancer
- PMID: 38580683
- PMCID: PMC10997660
- DOI: 10.1038/s41467-024-47002-3
Concomitant medication, comorbidity and survival in patients with breast cancer
Abstract
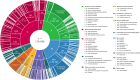
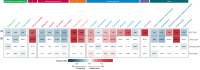
Between 30% and 70% of patients with breast cancer have pre-existing chronic conditions, and more than half are on long-term non-cancer medication at the time of diagnosis. Preliminary epidemiological evidence suggests that some non-cancer medications may affect breast cancer risk, recurrence, and survival. In this nationwide cohort study, we assessed the association between medication use at breast cancer diagnosis and survival. We included 235,368 French women with newly diagnosed non-metastatic breast cancer. In analyzes of 288 medications, we identified eight medications positively associated with either overall survival or disease-free survival: rabeprazole, alverine, atenolol, simvastatin, rosuvastatin, estriol (vaginal or transmucosal), nomegestrol, and hypromellose; and eight medications negatively associated with overall survival or disease-free survival: ferrous fumarate, prednisolone, carbimazole, pristinamycin, oxazepam, alprazolam, hydroxyzine, and mianserin. Full results are available online from an interactive platform ( https://adrenaline.curie.fr ). This resource provides hypotheses for drugs that may naturally influence breast cancer evolution.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

