Limited oxygen in standard cell culture alters metabolism and function of differentiated cells
- PMID: 38580776
- PMCID: PMC11148168
- DOI: 10.1038/s44318-024-00084-7
Limited oxygen in standard cell culture alters metabolism and function of differentiated cells
Erratum in
-
Author Correction: Limited oxygen in standard cell culture alters metabolism and function of differentiated cells.EMBO J. 2024 Oct;43(19):4439. doi: 10.1038/s44318-024-00230-1. EMBO J. 2024. PMID: 39242789 Free PMC article.
Abstract
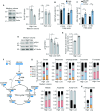
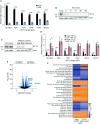
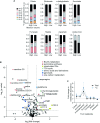
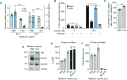
The in vitro oxygen microenvironment profoundly affects the capacity of cell cultures to model physiological and pathophysiological states. Cell culture is often considered to be hyperoxic, but pericellular oxygen levels, which are affected by oxygen diffusivity and consumption, are rarely reported. Here, we provide evidence that several cell types in culture actually experience local hypoxia, with important implications for cell metabolism and function. We focused initially on adipocytes, as adipose tissue hypoxia is frequently observed in obesity and precedes diminished adipocyte function. Under standard conditions, cultured adipocytes are highly glycolytic and exhibit a transcriptional profile indicative of physiological hypoxia. Increasing pericellular oxygen diverted glucose flux toward mitochondria, lowered HIF1α activity, and resulted in widespread transcriptional rewiring. Functionally, adipocytes increased adipokine secretion and sensitivity to insulin and lipolytic stimuli, recapitulating a healthier adipocyte model. The functional benefits of increasing pericellular oxygen were also observed in macrophages, hPSC-derived hepatocytes and cardiac organoids. Our findings demonstrate that oxygen is limiting in many terminally-differentiated cell types, and that considering pericellular oxygen improves the quality, reproducibility and translatability of culture models.
Keywords: Cell Culture; Hypoxia; Metabolism/Adipocytes; Oxygen Tension.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Abo KM, Sainz de Aja J, Lindstrom-Vautrin J, Alysandratos K-D, Richards A, Garcia-de-Alba C, Huang J, Hix OT, Werder RB, Bullitt E et al (2022) Air-liquid interface culture promotes maturation and allows environmental exposure of pluripotent stem cell-derived alveolar epithelium. JCI Insight 7:e155589 10.1172/jci.insight.155589 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- FS/17/61/33473/British Heart Foundation (BHF)
- New-Chol/EC | ERC | HORIZON EUROPE European Research Council (ERC)
- Senior Clinical Research Fellowship/Wellcome Trust (WT)
- BB/W005905/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- PhD Studentship/UKRI | Medical Research Council (MRC)
- BB/F016581/1/UKRI | Biotechnology and Biological Sciences Research Council (BBSRC)
- MRC_MC_UU_12022/6/UKRI | Medical Research Council (MRC)
- Snow Medical Fellowship/Snow Medical (SnowMedical)
- 204845/Z/16/Z/Wellcome Trust (WT)
- WT_/Wellcome Trust/United Kingdom
- PhD Studentship/Wellcome Trust (WT)
- MR/S007091/1/UKRI | Medical Research Council (MRC)
- Sir Henry Dale Fellowship/Wellcome Trust (WT)
- DOD-W81XWH-19-1-0213/DOD | Department of Defense Education Activity (DoDEA)
- MC_UU_00014/5/MRC_/Medical Research Council/United Kingdom
- RG/18/7/33636/British Heart Foundation (BHF)
- MC_UU_12012/2/UKRI | Medical Research Council (MRC)
LinkOut - more resources
Full Text Sources

