The role of vasculature and angiogenesis in respiratory diseases
- PMID: 38580869
- PMCID: PMC11303512
- DOI: 10.1007/s10456-024-09910-2
The role of vasculature and angiogenesis in respiratory diseases
Abstract
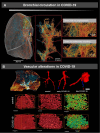
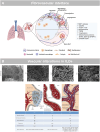
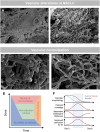
In European countries, nearly 10% of all hospital admissions are related to respiratory diseases, mainly chronic life-threatening diseases such as COPD, pulmonary hypertension, IPF or lung cancer. The contribution of blood vessels and angiogenesis to lung regeneration, remodeling and disease progression has been increasingly appreciated. The vascular supply of the lung shows the peculiarity of dual perfusion of the pulmonary circulation (vasa publica), which maintains a functional blood-gas barrier, and the bronchial circulation (vasa privata), which reveals a profiled capacity for angiogenesis (namely intussusceptive and sprouting angiogenesis) and alveolar-vascular remodeling by the recruitment of endothelial precursor cells. The aim of this review is to outline the importance of vascular remodeling and angiogenesis in a variety of non-neoplastic and neoplastic acute and chronic respiratory diseases such as lung infection, COPD, lung fibrosis, pulmonary hypertension and lung cancer.
Keywords: COPD; COVID-19; ECFC; Endothelial mesenchymal transition (EndoMT); Fibrovascular interface; Intussusceptive angiogenesis; Pulmonary fibrosis; Respiratory diseases; Tumor angiogenesis; Vascular normalization.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- WHO. World Health Statistics 2014 Date last updated May 15 2014. Available from: https://www.who.int/news/item/15-05-2014-world-health-statistics-2014. Date last accessed: November 22 2023
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

