The metabolic reprogramming of γ-aminobutyrate in oral squamous cell carcinoma
- PMID: 38580938
- PMCID: PMC10996254
- DOI: 10.1186/s12903-024-04174-0
The metabolic reprogramming of γ-aminobutyrate in oral squamous cell carcinoma
Abstract
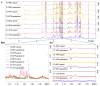
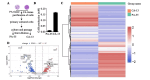
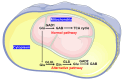
Oral squamous cell carcinoma (OSCC) is the most common head and neck malignancy. The oncometabolites have been studied in OSCC, but the mechanism of metabolic reprogramming remains unclear. To identify the potential metabolic markers to distinguish malignant oral squamous cell carcinoma (OSCC) tissue from adjacent healthy tissue and study the mechanism of metabolic reprogramming in OSCC. We compared the metabolites between cancerous and paracancerous tissues of OSCC patients by 1HNMR analysis. We established OSCC derived cell lines and analyzed their difference of RNA expression by RNA sequencing. We investigated the metabolism of γ-aminobutyrate in OSCC derived cells by real time PCR and western blotting. Our data revealed that much more γ-aminobutyrate was produced in cancerous tissues of OSCC patients. The investigation based on OSCC derived cells showed that the increase of γ-aminobutyrate was promoted by the synthesis of glutamate beyond the mitochondria. In OSCC cancerous tissue derived cells, the glutamate was catalyzed to glutamine by glutamine synthetase (GLUL), and then the generated glutamine was metabolized to glutamate by glutaminase (GLS). Finally, the glutamate produced by glutamate-glutamine-glutamate cycle was converted to γ-aminobutyrate by glutamate decarboxylase 2 (GAD2). Our study is not only benefit for understanding the pathological mechanisms of OSCC, but also has application prospects for the diagnosis of OSCC.
Keywords: Metabolic reprogramming; Oral squamous cell carcinoma; γ-aminobutyrate.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

