Cancer immunometabolism: advent, challenges, and perspective
- PMID: 38581001
- PMCID: PMC10996263
- DOI: 10.1186/s12943-024-01981-5
Cancer immunometabolism: advent, challenges, and perspective
Abstract
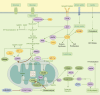
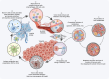
For decades, great strides have been made in the field of immunometabolism. A plethora of evidence ranging from basic mechanisms to clinical transformation has gradually embarked on immunometabolism to the center stage of innate and adaptive immunomodulation. Given this, we focus on changes in immunometabolism, a converging series of biochemical events that alters immune cell function, propose the immune roles played by diversified metabolic derivatives and enzymes, emphasize the key metabolism-related checkpoints in distinct immune cell types, and discuss the ongoing and upcoming realities of clinical treatment. It is expected that future research will reduce the current limitations of immunotherapy and provide a positive hand in immune responses to exert a broader therapeutic role.
Keywords: Cancer-immunity cycle; Cancer-immunometabolism subcycle; Immunity; Immunometabolism; Metabolic adaptation; Metabolic reprogramming.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Warburg O, Posener K, Negelein E. On the metabolism of carcinoma cells [J] Biochem Z. 1924;152:309–44.
-
- Dey P, Kimmelman AC, Depinho RA. Metabolic Codependencies in the Tumor Microenvironment [J] Cancer Discov. 2021;11(5):1067–81. doi: 10.1158/2159-8290.CD-20-1211. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

