How to differentiate induced pluripotent stem cells into sensory neurons for disease modelling: a functional assessment
- PMID: 38581069
- PMCID: PMC10998320
- DOI: 10.1186/s13287-024-03696-2
How to differentiate induced pluripotent stem cells into sensory neurons for disease modelling: a functional assessment
Abstract
Background: Human induced pluripotent stem cell (iPSC)-derived peripheral sensory neurons present a valuable tool to model human diseases and are a source for applications in drug discovery and regenerative medicine. Clinically, peripheral sensory neuropathies can result in maladies ranging from a complete loss of pain to severe painful neuropathic disorders. Sensory neurons are located in the dorsal root ganglion and are comprised of functionally diverse neuronal types. Low efficiency, reproducibility concerns, variations arising due to genetic factors and time needed to generate functionally mature neuronal populations from iPSCs remain key challenges to study human nociception in vitro. Here, we report a detailed functional characterization of iPSC-derived sensory neurons with an accelerated differentiation protocol ("Anatomic" protocol) compared to the most commonly used small molecule approach ("Chambers" protocol). Anatomic's commercially available RealDRG™ were further characterized for both functional and expression phenotyping of key nociceptor markers.
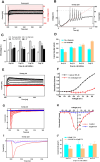
Methods: Multiple iPSC clones derived from different reprogramming methods, genetics, age, and somatic cell sources were used to generate sensory neurons. Manual patch clamp was used to functionally characterize both control and patient-derived neurons. High throughput techniques were further used to demonstrate that RealDRGs™ derived from the Anatomic protocol are amenable to high throughput technologies for disease modelling.
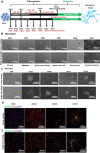
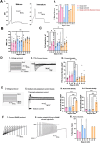
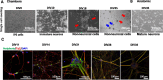
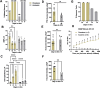
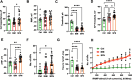
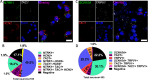
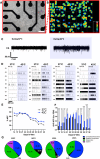
Results: The Anatomic protocol rendered a purer culture without the use of mitomycin C to suppress non-neuronal outgrowth, while Chambers differentiations yielded a mix of cell types. Chambers protocol results in predominantly tonic firing when compared to Anatomic protocol. Patient-derived nociceptors displayed higher frequency firing compared to control subject with both, Chambers and Anatomic differentiation approaches, underlining their potential use for clinical phenotyping as a disease-in-a-dish model. RealDRG™ sensory neurons show heterogeneity of nociceptive markers indicating that the cells may be useful as a humanized model system for translational studies.
Conclusions: We validated the efficiency of two differentiation protocols and their potential application for functional assessment and thus understanding the disease mechanisms from patients suffering from pain disorders. We propose that both differentiation methods can be further exploited for understanding mechanisms and development of novel treatments in pain disorders.
Keywords: Disease modelling; Human induced pluripotent stem cells; Pain; Sensory neurons; Sodium channel.
© 2024. The Author(s).
Conflict of interest statement
A.L. has a research agreement with Grunenthal. T.J.P has a research agreement with Grunenthal and Merck. A.L. and T.J.P. receive counselling fees from Grunenthal. P.W. and V.T. are shareholders and employees of Anatomic Incorporated. A.K.K. is currently employed at Grunenthal.
Figures


Update of
-
How to differentiate induced pluripotent stem cells into sensory neurons for disease modelling: a comparison of two protocols.Res Sq [Preprint]. 2023 Oct 28:rs.3.rs-3127017. doi: 10.21203/rs.3.rs-3127017/v1. Res Sq. 2023. Update in: Stem Cell Res Ther. 2024 Apr 5;15(1):99. doi: 10.1186/s13287-024-03696-2. PMID: 37961300 Free PMC article. Updated. Preprint.
References
-
- Gorecka J, Kostiuk V, Fereydooni A, Gonzalez L, Luo J, Dash B, Isaji T, Ono S, Liu S, Lee SR, Xu J, Liu J, Taniguchi R, Yastula B, Hsia HC, Qyang Y, Dardik A. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res Ther. 2019;10(1):87. doi: 10.1186/s13287-019-1185-1. - DOI - PMC - PubMed
-
- Cox JJ, Woods CG, Kurth I. Peripheral sensory neuropathies—pain loss vs. pain gain. Med Gen. 2020;32(3):233–241. doi: 10.1515/medgen-2020-2039. - DOI
-
- Renthal W, Chamessian A, Curatolo M, Davidson S, Burton M, Dib-Hajj S, Dougherty PM, Ebert AD, Gereau RW, 4th, Ghetti A, Gold MS, Hoben G, Menichella DM, Mercier P, Ray WZ, Salvemini D, Seal RP, Waxman S, Woolf CJ, Stucky CL, Price TJ. Human cells and networks of pain: Transforming pain target identification and therapeutic development. Neuron. 2021;109(9):1426–1429. doi: 10.1016/j.neuron.2021.04.005. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

