Genomic ancestry and cancer among Latin Americans
- PMID: 38581481
- PMCID: PMC11249489
- DOI: 10.1007/s12094-024-03415-6
Genomic ancestry and cancer among Latin Americans
Abstract
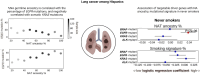
Latin American populations, characterized by intricate admixture patterns resulting from the intermingling of ancestries from European, Native American (NA) Asian, and African ancestries which result in a vast and complex genetic landscape, harboring unique combinations of novel variants. This genetic diversity not only poses challenges in traditional population genetics methods but also opens avenues for a deeper understanding of its implications in health. In cancer, the interplay between genetic ancestry, lifestyle factors, and healthcare disparities adds a layer of complexity to the varying incidence and mortality rates observed across different Latin American subpopulations. This complex interdependence has been unveiled through numerous studies, whether conducted on Latin American patients residing on the continent or abroad, revealing discernible differences in germline composition that influence divergent disease phenotypes such as higher incidence of Luminal B and Her2 breast tumors, EGFR and KRAS mutated lung adenocarcinomas in addition to an enrichment in BRCA1/2 pathogenic variants and a higher than expected prevalence of variants in colorectal cancer associated genes such as APC and MLH1. In prostate cancer novel risk variants have also been solely identified in Latin American populations. Due to the complexity of genetic divergence, inputs from each individual ancestry seem to carry independent contributions that interplay in the development of these complex disease phenotypes. By understanding these unique population characteristics, genomic ancestries hold a promising avenue for tailoring prognostic assessments and optimizing responses to oncological interventions.
Keywords: Cancer; Genetic ancestry; Genetic epidemiology; Genome‐wide association study; NA (NA); Polygenic risk score.
© 2024. The Author(s).
Conflict of interest statement
Jairo Zuluaga received honoraria as an advisor and participated in speakers' bureau of Astra Zeneca, Bristol Myers Squibb, and Pfizer. Leonardo Rojas received honoraria as an advisor and participated in speakers' bureau from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Astra Zeneca, and Eli Lilly. Additionally, he was linked and received honoraria as a researcher. Oscar Arrieta reports personal fees from Pfizer, grants and individual fees from Astra Zeneca, grants, and individual fees from Boehringer- Ingelheim, personal fees from Lilly, individual fees from Merck, personal fees from Bristol Myers Squibb, grants, and personal fees from Roche, outside the submitted work. Andrés F. Cardona discloses financial research support from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, and The Foundation for Clinical and Applied Cancer Research – FICMAC. Additionally, he was linked and received honoraria as an advisor, participated in the speakers' bureau, and gave expert testimony to Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly, and Foundation for Clinical and Applied Cancer Research – FICMAC.
Figures


References
-
- Chakraborty R. Gene admixture in human populations: models and predictions. Am J Phys Anthropol. 1986;29(S7):1–43.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

