Expanding the Scope of Aluminum Chemistry with Noninnocent Ligands
- PMID: 38581655
- PMCID: PMC11025028
- DOI: 10.1021/acs.accounts.3c00714
Expanding the Scope of Aluminum Chemistry with Noninnocent Ligands
Abstract
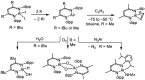
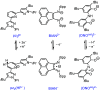
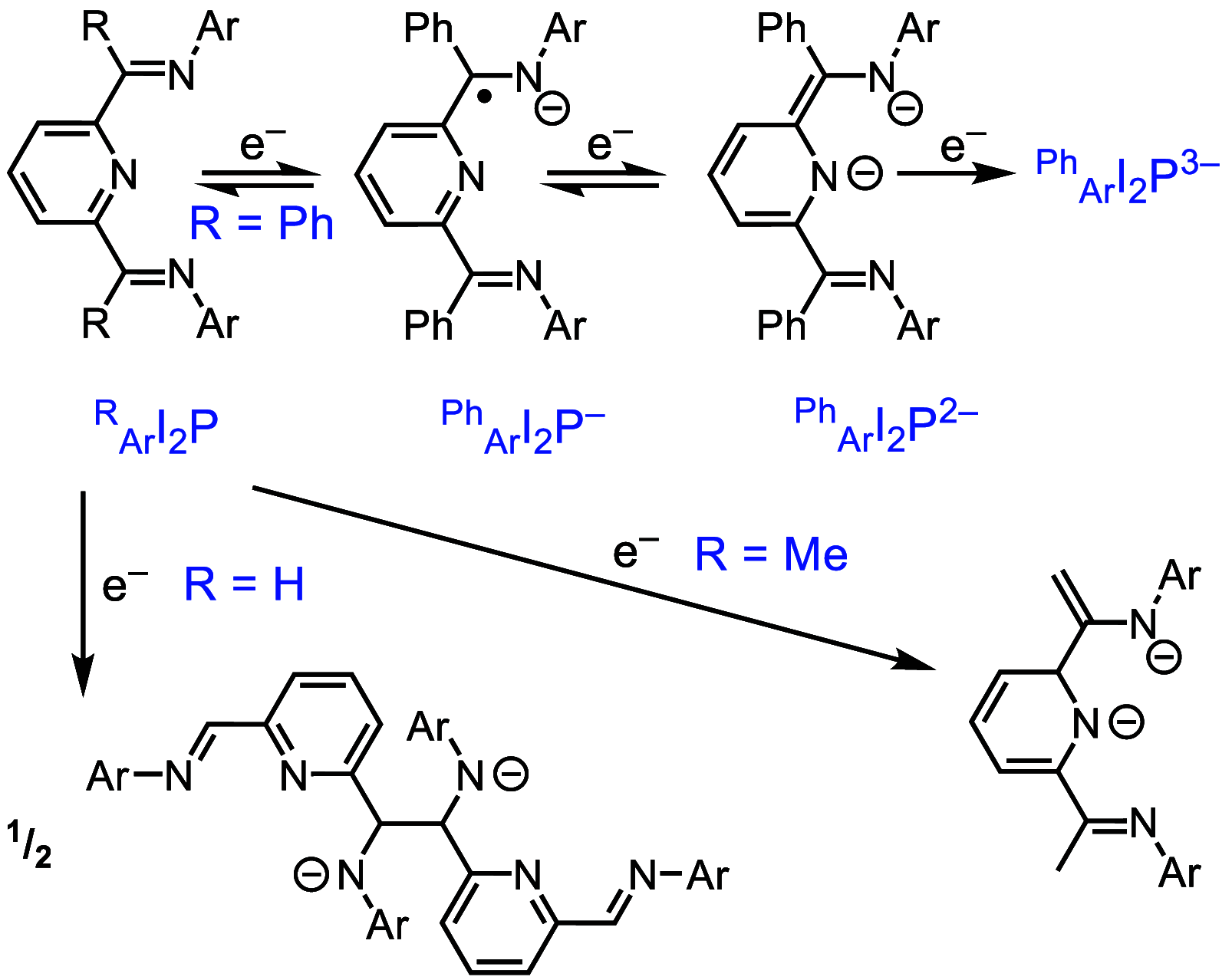
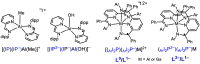
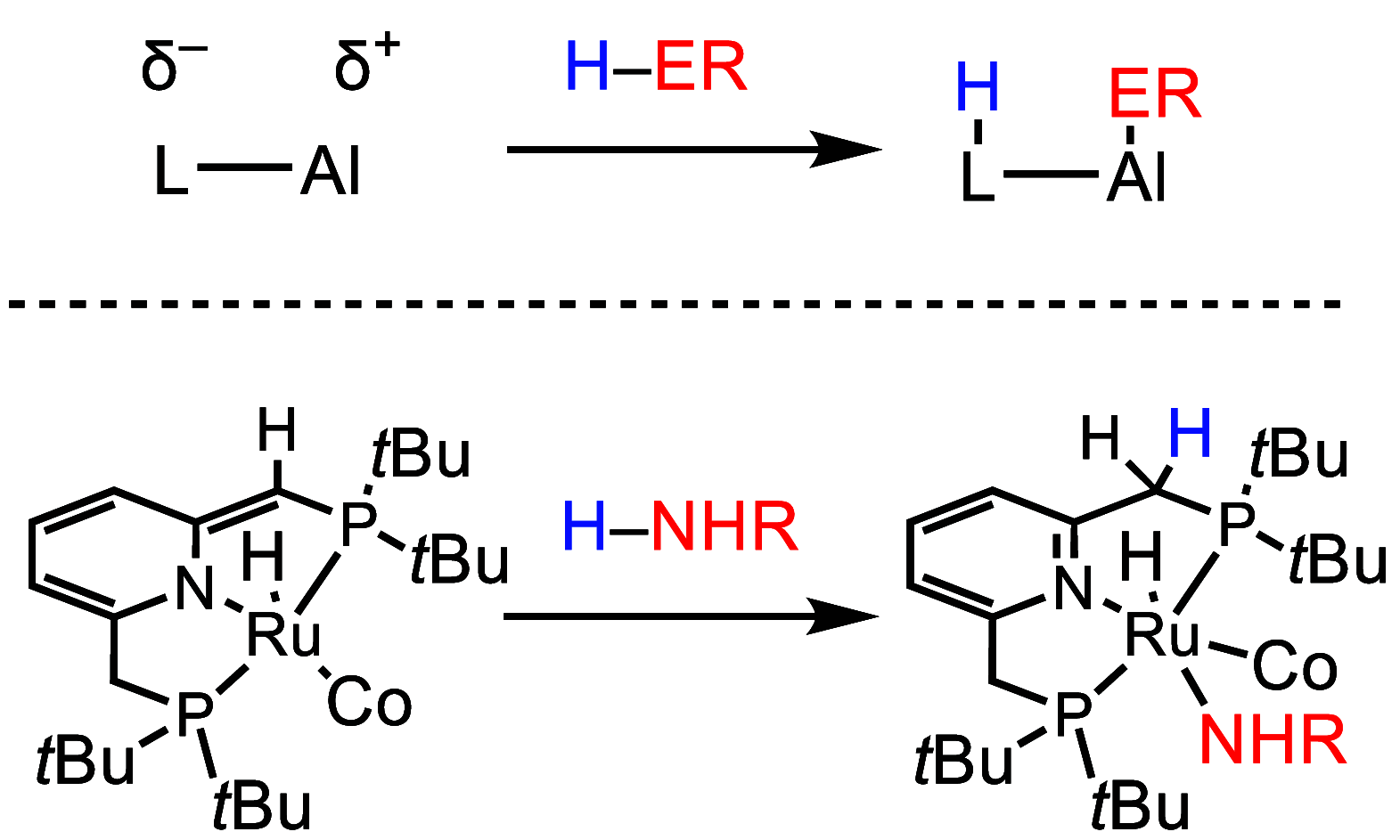
ConspectusAluminum is the most abundant metal in the earth's crust at 8%, and it is also widely available domestically in many countries worldwide, which ensures a stable supply chain. To further the applications of aluminum (Al), such as in catalysis and electronic and energy storage materials, there has been significant interest in the synthesis and characterization of new Al coordination compounds that can support electron transfer (ET) and proton transfer (PT) chemistry. This has been achieved using redox and chemically noninnocent ligands (NILs) combined with the highly stable M(III) oxidation state of Al and in some cases the heavier group 13 ions, Ga and In.When ligands participate in redox chemistry or facilitate the breaking or making of new bonds, they are often termed redox or chemically noninnocent, respectively. Al(III) in particular supports rich ligand-based redox chemistry because it is so redox inert and will support the ligand across many charge and protonation states without entering into the reaction chemistry. To a lesser extent, we have reported on the heavier group 13 elements Ga and In, and this chemistry will also be included in this Account, where available.This Account is arranged into two technical sections, which are (1) Structures of Al-NIL complexes and (2) Reactivity of Al-NIL complexes. Highlights of the research work include reversible redox chemistry that has been enabled by ligand design to shut down radical coupling pathways and to prevent loss of H2 from unsaturated ligand sites. These reversible redox properties have in turn enabled the characterization of Class III electron delocalization through Al when two NIL are bound to the Al(III) in different charge states. Characterization of the metalloaromatic character of square planar Al and Ga complexes has been achieved, and characterization of the delocalized electronic structures has provided a model within which to understand and predict the ET and PT chemistry of the NIL group 13 compounds. The capacity of Al-NIL complexes to perform ET and PT has been employed in reactions that use ET or PT reactivity only or in reactions where coupled ET/PT affords hydride transfer chemistry. As an example, ligand-based PT reactions initiate metal-ligand cooperative bond activation pathways for catalysis: this includes acceptorless dehydrogenation of formic acid and anilines and transfer hydrogenation chemistry. In a complementary approach, ligand based ET/PT chemistry has been used in the study of dihydropyridinate (DHP-) chemistry where it was shown that N-coordination of group 13 ions lowers kinetic barriers to DHP- formation. Taken together, the discussion presented herein illustrates that the NIL chemistry of Al(III), and also of Ga(III) and In(III) holds promise for further developments in catalysis and energy storage.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Carr C. R.; Vesto J. I.; Xing X.; Fettinger J. C.; Berben L. A. Aluminum–ligand cooperative O–H Bond Activation Initiates Catalytic Transfer Hydrogenation. ChemCatChem. 2022, 14, e202101869.10.1002/cctc.202101869. - DOI
-
- Weetman C.; Inoue S. The Road Travelled: After Main-Group Elements as Transition Metals. ChemCatChem. 2018, 10, 4213–4228. 10.1002/cctc.201800963. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

