Distinct subpopulations of human subcutaneous adipose tissue precursor cells revealed by single-cell RNA sequencing
- PMID: 38581663
- PMCID: PMC11193533
- DOI: 10.1152/ajpcell.00726.2023
Distinct subpopulations of human subcutaneous adipose tissue precursor cells revealed by single-cell RNA sequencing
Abstract
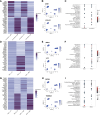
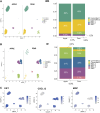
Adipose-derived stem cells (ADSCs) play an important role in the differential capacity for excess energy storage between upper body abdominal (ABD) adipose tissue (AT) and lower body gluteofemoral (GF) AT. We cultured ADSCs from subcutaneous ABD AT and GF AT isolated from eight women with differential body fat distribution and performed single-cell RNA sequencing. Six populations of ADSCs were identified and segregated according to their anatomical origin. The three ADSC subpopulations in GF AT were characterized by strong cholesterol/fatty acid (FA) storage and proliferation signatures. The two ABD subpopulations, differentiated by higher expression of committed preadipocyte marker genes, were set apart by differential expression of extracellular matrix and ribosomal genes. The last population, identified in both depots, was similar to smooth muscle cells and when individually isolated and cultured in vitro they differentiated less than the other subpopulations. This work provides important insight into the use of ADSC as an in vitro model of adipogenesis and suggests that specific subpopulations of GF-ADSCs contribute to the more robust capacity for GF-AT to expand and grow compared with ABD-AT in women.NEW & NOTEWORTHY Identification of distinct subpopulations of adipose-derived stem cells (ADSCs) in upper body abdominal subcutaneous (ABD) and lower body gluteofemoral subcutaneous (GF) adipose tissue depots. In ABD-ADSCs, subpopulations are more committed to adipocyte lineage. GF-ADSC subpopulations are enriched for genes involved in lipids and cholesterol metabolism. Similar depot differences were found in stem cell population identified in freshly isolated stoma vascular fraction. The repertoire of ADSCs subpopulations was different in apple-shaped versus pear-shaped women.
Keywords: abdominal adipose tissue; adipose-derived stem cells; cell identity; gluteofemoral adipose tissue; scRNA-seq.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 50: 425–435, 2001. doi: 10.1053/meta.2001.21693. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

