Spatial Architecture of Myeloid and T Cells Orchestrates Immune Evasion and Clinical Outcome in Lung Cancer
- PMID: 38581685
- PMCID: PMC11145179
- DOI: 10.1158/2159-8290.CD-23-1380
Spatial Architecture of Myeloid and T Cells Orchestrates Immune Evasion and Clinical Outcome in Lung Cancer
Abstract
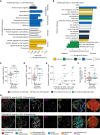
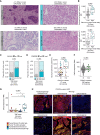
Understanding the role of the tumor microenvironment (TME) in lung cancer is critical to improving patient outcomes. We identified four histology-independent archetype TMEs in treatment-naïve early-stage lung cancer using imaging mass cytometry in the TRACERx study (n = 81 patients/198 samples/2.3 million cells). In immune-hot adenocarcinomas, spatial niches of T cells and macrophages increased with clonal neoantigen burden, whereas such an increase was observed for niches of plasma and B cells in immune-excluded squamous cell carcinomas (LUSC). Immune-low TMEs were associated with fibroblast barriers to immune infiltration. The fourth archetype, characterized by sparse lymphocytes and high tumor-associated neutrophil (TAN) infiltration, had tumor cells spatially separated from vasculature and exhibited low spatial intratumor heterogeneity. TAN-high LUSC had frequent PIK3CA mutations. TAN-high tumors harbored recently expanded and metastasis-seeding subclones and had a shorter disease-free survival independent of stage. These findings delineate genomic, immune, and physical barriers to immune surveillance and implicate neutrophil-rich TMEs in metastasis.
Significance: This study provides novel insights into the spatial organization of the lung cancer TME in the context of tumor immunogenicity, tumor heterogeneity, and cancer evolution. Pairing the tumor evolutionary history with the spatially resolved TME suggests mechanistic hypotheses for tumor progression and metastasis with implications for patient outcome and treatment. This article is featured in Selected Articles from This Issue, p. 897.
©2024 The Authors; Published by the American Association for Cancer Research.
Figures




References
-
- Ali HR, Jackson HW, Zanotelli VRT, Danenberg E, Fischer JR, Bardwell H, et al. . Imaging mass cytometry and multiplatform genomics define the phenogenomic landscape of breast cancer. Nat Cancer 2020;1:163–75. - PubMed
-
- Jackson HW, Fischer JR, Zanotelli VRT, Ali HR, Mechera R, Soysal SD, et al. . The single-cell pathology landscape of breast cancer. Nature 2020;578:615–20. - PubMed
MeSH terms
Grants and funding
- C11496/A17786/Cancer Research UK (CRUK)
- C416/A21999/Cancer Research UK (CRUK)
- BCRF-22-157/Breast Cancer Research Foundation (BCRF)
- 835297/ERC_/European Research Council/International
- MFCR ASPIRE 2022- 0384/Mark Foundation For Cancer Research (The Mark Foundation for Cancer Research)
- CTUQQR-DEC22/100009/CRUK_/Cancer Research UK/United Kingdom
- 20-45200-98-20102/ZonMw (Netherlands Organisation for Health Research and Development)
- 101079113/HORIZON EUROPE European Research Council (ERC)
- 23-034-ASP/Mark Foundation For Cancer Research (The Mark Foundation for Cancer Research)
- SU2C-AACR-DT23-17/Stand Up To Cancer (SU2C)
- 101019366/European Research Council (ERC)
- CC2041/CRUK_/Cancer Research UK/United Kingdom
- Amsterdam UMC Fellowship/Amsterdam University Medical Centers (AUMC)
- 211179/Z/18/Z/Wellcome Trust (WT)
- C11496/A30025/CRUK Lung Cancer Centre of Excellence (CRUKLungCentre)
- 211179/Z/18/Z/Royal Society (The Royal Society)
- 17786/CRUK_/Cancer Research UK/United Kingdom
- Grant EDDPMA-Nov21/100034/Cancer Research UK (CRUK)
- CC2040/WT_/Wellcome Trust/United Kingdom
- Rosetrees Trust (Rosetrees)
- UCLH Biomedical Research Centre (UCL)
- 30025/CRUK_/Cancer Research UK/United Kingdom
- CC2040/CRUK_/Cancer Research UK/United Kingdom
- 202060447/Japan Society for the Promotion of Science London (JSPS)
- 211179/WT_/Wellcome Trust/United Kingdom
- Grant 21-029-ASP/Mark Foundation For Cancer Research (The Mark Foundation for Cancer Research)
- CRUK Lung Cancer Centre of Excellence (CRUKLungCentre)
- RF\ERE\231118/Royal Society (The Royal Society)
- 101024529/HORIZON EUROPE Marie Sklodowska-Curie Actions (MSCA)
- RF\ERE\210216/Royal Society (The Royal Society)
- 838540/HORIZON EUROPE Marie Sklodowska-Curie Actions (MSCA)
- 835297/HORIZON EUROPE European Research Council (ERC)
- CRUK Cancer Immunotherapy Catalyst Network/Cancer Research UK (CRUK)
- CC2041/WT_/Wellcome Trust/United Kingdom
- ID16584/Novo Nordisk Foundation Center for Basic Metabolic Research (NovoNordisk Foundation Center for Basic Metabolic Research)
- RP/EA/180007/Royal Society (The Royal Society)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

