ALOX15B controls macrophage cholesterol homeostasis via lipid peroxidation, ERK1/2 and SREBP2
- PMID: 38581859
- PMCID: PMC11002893
- DOI: 10.1016/j.redox.2024.103149
ALOX15B controls macrophage cholesterol homeostasis via lipid peroxidation, ERK1/2 and SREBP2
Abstract
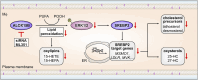
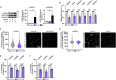
Macrophage cholesterol homeostasis is crucial for health and disease and has been linked to the lipid-peroxidizing enzyme arachidonate 15-lipoxygenase type B (ALOX15B), albeit molecular mechanisms remain obscure. We performed global transcriptome and immunofluorescence analysis in ALOX15B-silenced primary human macrophages and observed a reduction of nuclear sterol regulatory element-binding protein (SREBP) 2, the master transcription factor of cellular cholesterol biosynthesis. Consequently, SREBP2-target gene expression was reduced as were the sterol biosynthetic intermediates desmosterol and lathosterol as well as 25- and 27-hydroxycholesterol. Mechanistically, suppression of ALOX15B reduced lipid peroxidation in primary human macrophages and thereby attenuated activation of mitogen-activated protein kinase ERK1/2, which lowered SREBP2 abundance and activity. Low nuclear SREBP2 rendered both, ALOX15B-silenced and ERK1/2-inhibited macrophages refractory to SREBP2 activation upon blocking the NPC intracellular cholesterol transporter 1. These studies suggest a regulatory mechanism controlling macrophage cholesterol homeostasis based on ALOX15B-mediated lipid peroxidation and concomitant ERK1/2 activation.
Keywords: 15-LO2; Arachidonate 15-lipoxygenase type B; Lipid peroxidation; MAPK; Reactive oxygen species; Sterol regulatory element-binding protein 2.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures




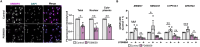
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

