HIV-1 Integrase Assembles Multiple Species of Stable Synaptic Complex Intasomes That Are Active for Concerted DNA Integration In vitro
- PMID: 38582148
- PMCID: PMC11134455
- DOI: 10.1016/j.jmb.2024.168557
HIV-1 Integrase Assembles Multiple Species of Stable Synaptic Complex Intasomes That Are Active for Concerted DNA Integration In vitro
Abstract
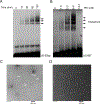
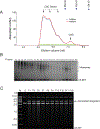
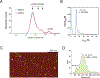
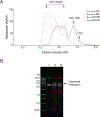
Retroviral DNA integration is mediated by nucleoprotein complexes (intasomes) in which a pair of viral DNA ends are bridged by a multimer of integrase (IN). Most of the high-resolution structures of HIV-1 intasomes are based on an HIV-1 IN with an Sso7d protein domain fused to the N-terminus. Sso7d-IN aggregates much less than wild-type IN and has been critical for structural studies of HIV-1 intasomes. Unexpectedly, these structures revealed that the common core architecture that mediates catalysis could be assembled in various ways, giving rise to both tetrameric and dodecameric intasomes, together with other less well-characterized species. This differs from related retroviruses that assemble unique multimeric intasomes, although the number of protomers in the intasome varies between viruses. The question of whether the additional Sso7d domain contributes to the heterogeneity of HIV-1 intasomes is therefore raised. We have addressed this by biochemical and structural studies of intasomes assembled with wild-type HIV-1 IN. Negative stain and cryo-EM reveal a similar range of multimeric intasome species as with Sso7d-IN with the same common core architecture. Stacks of intasomes resulting from domain swapping are also seen with both wild-type and Sso7d-IN intasomes. The propensity to assemble multimeric intasome species is, therefore, an intrinsic property of HIV-1 IN and is not conferred by the presence of the Sso7d domain. The recently solved intasome structures of different retroviral species, which have been reported to be tetrameric, octameric, dodecameric, and hexadecameric, highlight how a common intasome core architecture can be assembled in different ways for catalysis.
Keywords: HIV; integrase; integration; nucleoprotein complex; retrovirus.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
HIV-1 Intasomes Assembled with Excess Integrase C-Terminal Domain Protein Facilitate Structural Studies by Cryo-EM and Reveal the Role of the Integrase C-Terminal Tail in HIV-1 Integration.Viruses. 2024 Jul 20;16(7):1166. doi: 10.3390/v16071166. Viruses. 2024. PMID: 39066328 Free PMC article.
-
Cryo-EM structures and atomic model of the HIV-1 strand transfer complex intasome.Science. 2017 Jan 6;355(6320):89-92. doi: 10.1126/science.aah5163. Science. 2017. PMID: 28059769 Free PMC article.
-
Outer domains of integrase within retroviral intasomes are dispensible for catalysis of DNA integration.Protein Sci. 2016 Feb;25(2):472-8. doi: 10.1002/pro.2837. Epub 2015 Nov 25. Protein Sci. 2016. PMID: 26537415 Free PMC article.
-
Nucleoprotein Intermediates in HIV-1 DNA Integration: Structure and Function of HIV-1 Intasomes.Subcell Biochem. 2018;88:189-210. doi: 10.1007/978-981-10-8456-0_9. Subcell Biochem. 2018. PMID: 29900498 Free PMC article. Review.
-
Retroviral integrase protein and intasome nucleoprotein complex structures.World J Biol Chem. 2017 Feb 26;8(1):32-44. doi: 10.4331/wjbc.v8.i1.32. World J Biol Chem. 2017. PMID: 28289517 Free PMC article. Review.
Cited by
-
Oligomeric HIV-1 Integrase Structures Reveal Functional Plasticity for Intasome Assembly and RNA Binding.bioRxiv [Preprint]. 2025 May 21:2024.01.26.577436. doi: 10.1101/2024.01.26.577436. bioRxiv. 2025. PMID: 38328132 Free PMC article. Preprint.
-
HIV-1 Intasomes Assembled with Excess Integrase C-Terminal Domain Protein Facilitate Structural Studies by Cryo-EM and Reveal the Role of the Integrase C-Terminal Tail in HIV-1 Integration.Viruses. 2024 Jul 20;16(7):1166. doi: 10.3390/v16071166. Viruses. 2024. PMID: 39066328 Free PMC article.
References
-
- Engelman A, Mizuuchi K, Craigie R, (1991). HIV-1 DNA integration: mechanism of viral DNA cleavage and DNA strand transfer. Cell 67, 1211–1221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

